Propanc Provides Shareholder Update on PRP Development Activities
PRP Enters Formal Preclinical Development and Preparation for First-In-Man Studies
MELBOURNE, Australia, June 13, 2016 /PRNewswire/ -- Propanc Health Group Corporation (OTCQB: PPCH) ("Propanc" or "the Company"), an emerging healthcare company focusing on development of new and proprietary treatments for cancer patients suffering from solid tumors such as pancreatic, ovarian and colorectal cancers, today announced an update on the significant progress of PRP development activities towards First-in-Man studies since the successful completion of the Medicines and Healthcare Products Regulatory Agency (MHRA) meeting in London, UK, April 25th.
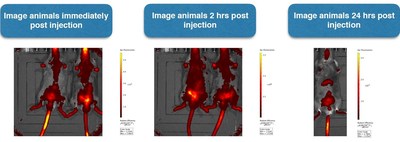
Since the meeting, the Company initiated a number of activities relating to quality and manufacturing of raw materials and finished drug product fill for clinical trials, and non-clinical development of PRP. Non clinical activities include pharmacokinetic (measurement of movement and distribution of drug in the blood) method development of PRP in blood plasma and an upcoming formal 28 day toxicology study (including a toxicokinetic arm, designed to determine the relationship between the level of exposure of PRP in the blood and its toxicity).
These activities include:
- Detailed discussions held with Quality Manufacturers regarding the purification of starting materials, characterization of the API's (Active Pharmaceutical Ingredients) and future planned upscaling of GMP (Good Manufacturing Practice) manufacture of API's, as well as finished product manufacture of PRP in sufficient quantities to cover planned Phase I patient trials;
- Commencement of method development with an analytical services laboratory in the UK, developing the scientific methods required to characterize the raw materials prior to and completion of the purification process of API materials for manufacturing;
- Initiation of a validation study for an IR (infrared) dye-labelled detection method for both, trypsinogen and chymotrypsinogen measuring bio-distribution in blood plasma and tissue samples for an upcoming 28 day toxicokinetic study. Importantly, results so far indicate a faster clearance rate than expected and there is strong support for daily dosing of both compounds;
- Development work for an ELISA (enzyme linked immunosorbent assay) conducted with a research institution in Melbourne, Australia, to generate polyclonal antibodies against the two proenzymes, trypsinogen and chymotrypsinogen, as well as the two enzymes activated from the proenzymes, for clinical trials. Initial antibody titers are very encouraging.
- A recent test facility audit successfully completed at vivoPharm Australia in preparation for the upcoming 28 day repeated dose toxicology study to be conducted in the third quarter this year in Melbourne, Australia.
- Sufficient quantities of raw materials of trypsinogen and chymotrypsinogen sourced from the Company's current supplier and soon to be released for the upcoming 28 day study and planned API purification process and finished product manufacturing of PRP.
"Over the last six months, Propanc's development team has worked extremely hard to progress PRP to a stage where a constructive discussion could be held with the MHRA to define our development pathway," said James Nathanielsz, Propanc's Chief Executive Officer. "I am pleased to say we had a successful meeting and are working hard to complete the activities necessary to support a clinical trial application. So far, it appears we are on track to complete this goal early next year and I am delighted with the level of commitment and effort shown by our Development team. As I mentioned previously, we are presently transforming into a clinical stage biopharmaceutical company and I expect more exciting developments to unfold this year as we progress towards first-in-man studies for PRP."
In further news, the Company has commenced preparing orphan drug designation (ODD) applications for treatment of pancreatic and ovarian cancers to the European Medicines Agency (EMA) and US Food and Drug Administration (FDA) during the second half of 2016. Upcoming licensing discussions with strategic partners are also expected to take place later this year.
The Company aims to fast track the development of proenzyme related oncology products into clinical trials initially for pancreatic and ovarian cancers, followed by colorectal cancer. According to Global Analyst Reports, the combined world market for pancreatic, ovarian and colorectal cancers are expected to reach over $12 billion by 2020.
About Propanc:
Propanc is developing new cancer treatments for patients suffering from pancreatic, ovarian and colorectal cancers. We have developed a formulation of anti-cancer compounds, which exert a number of effects designed to control or prevent tumors from recurring and spreading throughout the body. Our products involve or employ pancreatic proenzymes, which are inactive precursors of enzymes.
In the near term, we intend to target patients with limited remaining therapeutic options for the treatment of solid tumors. In future, we intend to develop our lead product to treat (i) early stage cancer and (ii) pre-cancerous diseases and (iii) as a preventative measure for patients at risk of developing cancer based on genetic screening. For more information, visit: www.propanc.com
Forward-looking Statements:
Certain of the matters discussed in this announcement involve risks and uncertainties including, without limitation, those regarding the Company's ability to establish and maintain the proprietary nature of its technology through the patent process, its ability to license from others patents and patent applications, if necessary, to develop certain products, its ability to implement its long range business plan for various applications of its technology, and its ability to enter into agreements with any necessary marketing and/or distribution partners for purposes of commercialization. This is not a solicitation to buy or sell securities and does not purport to be an analysis of the company's financial position. See Propanc's most recent Quarterly Report on Form 10-Q and related 8K filings.

Photo - https://photos.prnewswire.com/prnh/20160610/377834
Logo - https://photos.prnewswire.com/prnh/20160429/361516LOGO
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/propanc-provides-shareholder-update-on-prp-development-activities-300282921.html
SOURCE Propanc Health Group Corporation
Released June 13, 2016
