Propanc Biopharma Analyzes Pancreatic Proenzymes Vs T-Cell Therapy Targeting Solid Tumors Such as Pancreatic, Ovarian & Colorectal Cancers
MELBOURNE, Australia--(BUSINESS WIRE)-- Propanc Biopharma, Inc. (OTC: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today analyzes pancreatic proenzymes versus T-Cell therapy when targeting solid tumors such as pancreatic, ovarian and colorectal cancers. The analysis is prepared by the Company’s Chief Executive Officer, Mr. James Nathanielsz, in collaboration with joint lead researcher, Professor Macarena Perán, from the University of Jaén, Granada, Spain.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210111005376/en/
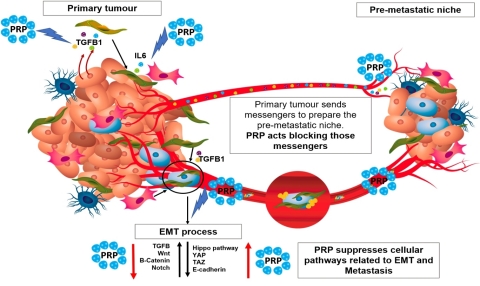
Cell differentiation therapy using pancreatic proenzymes has shown to degrade the fibrotic tissue on the surface of solid tumors and therefore might impair tumor engrafting, tumor niche formation and even cancer stem cell subpopulation activation. (Photo: Business Wire)
“We are making significant inroads in the way we treat cancer today, but there is a genuine need to continually challenge ourselves to improve the standard of care for many cancer types,” said James Nathanielsz. “At Propanc, we share a vision to develop and commercialize a novel approach using pancreatic proenzymes for the long-term treatment and prevention of metastatic cancer from solid tumors. Our goal is to reduce the threat of cancer by extending life meaningfully, but not at the expense of great toxicity. This humanitarian cause affects us all.”
Everybody knows what cancer is. Cells in the body begin to divide rapidly and uncontrollably in the body, with an ability to migrate from one location and spread to distant sites. However, when a cell becomes undifferentiated, forgetting how to do its job and investing all its energy in proliferating, it becomes cancerous. Unlike normal cells, cancer cells multiply, but do not differentiate. Most common therapies take advantage of the uncontrolled proliferation and kill these cells by targeting the cell division machinery. These therapies are effective, but affect healthy cells as well, particularly those with a high cell turn over, inducing undesirable effects. More recently, scientific advancements have meant that T-cell therapies are considered a tremendous improvement compared to older treatments. T-cell therapy involves using specific T-cells from the patient’s own immune system. Doctors take a type of white blood cell from the patient’s body and genetically change the cells in a lab so they can better find the cancer. Then millions of these target-seeking cells are put back into the patient.
The use of cancer-specific T-cells is a clever strategy to use the natural weapons from the body against cancer cells. This is a genuine targeted therapy, which kills cancer by recognizing antigen targets expressed on the cancer cell surface. This novel strategy is promising, although it still has some challenges. Of most importance is the health of patient's T-cells, which may decline due to age, or degeneration induced by the cancer itself, which is not ideal. There are also limitations with regards to efficacy and safety, and they are highly expensive. Resistance can develop over time, as specific antigens mutate, causing tumor escape and disease relapse. Furthermore, a patient can have serious side effects, including very high fevers and dangerously low blood pressure days after treatment. Other serious side effects include neurotoxicity, or changes in the brain that cause swelling, confusion, seizures, or severe headaches. Another problem is that T-cells can kill off some of the good B-cells that help fight germs, so the patient may be at higher risk for infection. Finally, when factoring in all the costs associated with T-cell therapies, hospitals may charge as much as $1.5 million or more to avoid losing money.
So, whilst enhancing a patient’s immune response to attack cancer has genuine merit, other ways to stop cancer are needed to further reduce the threat of cancer from a killer disease to a chronic (long term) illness. Another approach to stop cancer is not by targeting cell death, but inducing cell differentiation. This is known as cell differentiation therapy. The key consideration is how to convince the malignant cells to stop proliferating and return to their role as a specific cell type.
So, what are the advantages of cell differentiation therapy over other strategies, like T-Cell therapy? Firstly, cell differentiation therapy does not target cell death, so healthy cells are not compromised. Cell differentiation therapy induces cancer cells to differentiate and become non-proliferative (non-replicating), so they die naturally. Cell differentiation therapy acts not only against cancer cells, but interestingly can turn cancer stem cells (undifferentiated cells) towards completely differentiated, i.e., normal cells. Significantly, once the cancer stem cells are completely differentiated, they are no longer hidden from the immune system. This means that the body’s immune response can more effectively target the cancer, and therefore, in theory, will be complementary to immunological approaches like T-Cell therapy, by improving response rates and reducing toxicity.
More than 100 years ago, a comparative embryologist Professor John Beard first proposed that pancreatic enzymes represent the body’s primary defense against cancer and would prove useful as a cancer treatment. Since then, scientists have endorsed Beard’s hypothesis with encouraging data from patient treatment. After extensive laboratory research over the last decade and limited human testing by compassionate use, there is evidence that pancreatic proenzymes reduces cancer cell growth via promotion of cell differentiation, enhances cell adhesion (cell to cell contact) and suppresses metastasis (cancer spread), has no serious side effects and improves patient survival. The unique approach targets and eradicate cancer stem cells, which can migrate to other organs triggering explosive tumor growth, causing the patient to relapse after standard treatments that do not target non-dividing cells. Eighty percent of cancers are from solid tumors and metastasis is the main cause of patient death, therefore the potential of cell differentiation therapy using pancreatic proenzymes is significant. Given they are derived from natural sources, pancreatic proenzymes are also not cost prohibitive.
There is little doubt that both the T-Cell based and cell differentiation therapy approaches have emerged to address the limited efficacy of chemotherapy and radiation therapy for patients with advanced solid tumors. Although both therapies bear a slight resemblance because they enhance the immune response, they are not comparable by their mode of action. It is also understood the tumor micro-environment promotes the appearance of new cancer stem cells, derived from non-stem cancerous cells by secreting several biomarkers, such as IL6 (interleukin 6), HGF (hepatocyte growth factor), or TGFβ-163 (tumor growth factor beta-163). Consequently, it is critical to impact the tumor micro-environment in order to effectively eradicate the tumor. Cell differentiation therapy using pancreatic proenzymes has shown to degrade the fibrotic tissue on the surface of solid tumors and therefore might impair tumor engrafting, tumor niche formation and even cancer stem cell subpopulation activation.
Whilst T-Cell therapy has helped to advance the treatment of cancer, there are new and exciting approaches which are complementary and may provide a long-term solution to the treatment and prevention of metastatic cancer from most common solid tumors. Cell differentiation therapy using pancreatic proenzymes is based on the original work by John Beard, a professor of embryology at Edinburgh University over 100 years ago, using fresh pancreatic extracts. Through advancements in science and technology, there is an opportunity to introduce an improved version of this hypothesis, as a long-term therapeutic approach to treat metastatic cancer from solid tumors, which today, remains the main cause of patient death for sufferers.
Bibliography
- “Antitumor efficacy of chymotrypsinogen and trypsinogen,” P. Hernández, E. López-Ruiz, M. A. García, J. A. Marchal, J. Kenyon, M. Perán.
- “In vitro treatment of carcinoma cell lines with pancreatic (pro)enzymes suppresses the EMT programme and promotes cell differentiation”, M. Perán, J.A. Marchal, M.A. García, J. Kenyon & D. Tosh.
- “A formulation of pancreatic proenzymes provides potent anti-tumour efficacy: a pilot study focused on pancreatic and ovarian cancer”, M. Perán, E. López-Ruiz, M. A. García, S. Nadaraia-Hoke, R. Brandt, J. A. Marchal & J. Kenyon.
- “Pancreatic proenzymes treatment suppresses BXPC-3 pancreatic Cancer Stem Cell subpopulation and impairs tumour engrafting,” P. Hernández-Camarero, E. López-Ruiz, C. Griñán-Lisón, M.A. García, C. Chocarro-Wrona, J.A. Marchal, J. Kenyon & M. Perán.
- “Trypsinogen and Chymotrypsinogen: Potent Anti-Tumour Agents,” A. González-Titos, P. Hernández-Camarero, S. Barungi, J.A. Marchal, J. Kenyon & M. Perán. *
*Draft manuscript under review
About Propanc Biopharma, Inc.
Propanc Biopharma, Inc. (the “Company”) is developing a novel cell differentiation therapy using pancreatic proenzymes that target and eradicate cancer stem cells to prevent recurrence and metastasis of solid tumors in patients suffering from pancreatic, ovarian and colorectal cancers. For more information, please visit www.propanc.com.
The Company’s novel cell differentiation therapy is based on the science that enzymes stimulate biological reactions in the body, especially enzymes secreted by the pancreas. These pancreatic enzymes could represent the body’s primary defense against cancer.
To view the Company’s “Mechanism of Action” video on its anti-cancer lead product candidate, PRP, please click on the following link: http://www.propanc.com/news-media/video
Forward-Looking Statements
All statements other than statements of historical facts contained in this press release are “forward-looking statements,” which may often, but not always, be identified by the use of such words as “may,” “might,” “will,” “will likely result,” “would,” “should,” “estimate,” “plan,” “project,” “forecast,” “intend,” “expect,” “anticipate,” “believe,” “seek,” “continue,” “target” or the negative of such terms or other similar expressions. These statements involve known and unknown risks, uncertainties and other factors, which may cause actual results, performance or achievements to differ materially from those expressed or implied by such statements. These factors include uncertainties as to the Company’s ability to continue as a going concern absent new debt or equity financings; the Company’s current reliance on substantial debt financing that it is unable to repay in cash; the Company’s ability to successfully remediate material weaknesses in its internal controls; the Company’s ability to reach research and development milestones as planned and within proposed budgets; the Company’s ability to control costs; the Company’s ability to obtain adequate new financing on reasonable terms; the Company’s ability to successfully initiate and complete clinical trials and its ability to successful develop PRP, its lead product candidate; the Company’s ability to obtain and maintain patent protection; the Company’s ability to recruit employees and directors with accounting and finance expertise; the Company’s dependence on third parties for services; the Company’s dependence on key executives; the impact of government regulations, including FDA regulations; the impact of any future litigation; the availability of capital; changes in economic conditions, competition; and other risks, including, but not limited to, those described in the Company’s Registration Statement on Form S-1, Amendment No. 5, filed with the U.S. Securities and Exchange Commission (the “SEC”) on November 3, 2020, and in the Company’s other filings and submissions with the SEC. These forward-looking statements speak only as of the date hereof and the Company disclaims any obligations to update these statements except as may be required by law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210111005376/en/
Investor Relations and Media:
Mr. James Nathanielsz
Propanc Biopharma, Inc.
irteam@propanc.com
+61-3-9882-0780
Source: Propanc Biopharma, Inc.
Released January 11, 2021
