
As filed with the U.S. Securities and Exchange Commission on February 25, 2019.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Propanc Biopharma, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 2834 | 33-0662986 | ||
(State or other jurisdiction of incorporation) |
(Primary
Standard Industrial Classification Code Number) |
(I.R.S.
Employer Identification Number) |
James Nathanielsz
Chief Executive Officer
Propanc Biopharma, Inc.
302, 6 Butler Street
Camberwell, VIC, 3124 Australia
+ 61-03-9882-6723
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
James Nathanielsz
Chief Executive Officer
Propanc Biopharma, Inc.
302, 6 Butler Street
Camberwell, VIC, 3124 Australia
+ 61-03-9882-6723
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
Jonathan Shechter, Esq.
Sasha Ablovatskiy, Esq.
Foley Shechter Ablovatskiy LLP
211 East 43rd Street, Seventh Floor
New York, New York 10017
Tel.: (212) 335-0466
Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box: [X]
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier registration statement for the same offering. [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier registration statement for the same offering. [ ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer: [ ] | Accelerated filer: [ ] | |
| Non-accelerated filer [ ] | (Do not check if a smaller reporting company) | Smaller reporting company [X] |
| Emerging growth company [ ] | ||
CALCULATION OF REGISTRATION FEE
| Title
of Each Class of Securities to be Registered | Amount to be Registered (1) | Proposed Maximum Offering Price Per Share (2) | Proposed Maximum Aggregate Offering Price (2) | Amount
of Registration Fee | ||||||||||||
| Common Stock, $0.001 value per share | 111,203,954 | $ | 0.01429 | $ | 1,589,104.50 | $ | 192.60 | |||||||||
| (1) | An indeterminate number of additional shares of common stock shall be issuable pursuant to Rule 416 under the Securities Act of 1933, as amended (the “Securities Act”) to prevent dilution resulting from stock splits, stock dividends or similar transactions and in such an event the number of shares registered shall automatically be increased to cover the additional shares in accordance with Rule 416. | |
| (2) | Estimated solely for the purpose of calculating the amount of the registration fee in accordance with Rule 457(c) under the Securities Act of 1933, as amended, based on the last reported sale price of the Registrant’s common stock as reported on the OTC Markets - OTCQB on February 20, 2019. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
PRELIMINARY PROSPECTUS, SUBJECT TO COMPLETION, DATED FEBRUARY 25, 2019

Propanc Biopharma, Inc.
111,203,954 Shares of Common Stock
This prospectus relates to the offer and resale of up to 111,203,954 shares of our common stock, par value $0.001 per share, by the selling stockholder identified on page 28. Such shares represent the shares that Oasis Capital, LLC (“Oasis Capital”) has agreed to purchase from us pursuant to the terms and conditions of an Equity Purchase Agreement that we entered into with Oasis Capital on February 25, 2019 (the “Equity Purchase Agreement”). Subject to the terms and conditions of the Equity Purchase Agreement, we have the right to “put,” or sell, at our discretion, up to $10,000,000 worth of shares of our common stock to Oasis Capital. This arrangement is also sometimes referred to herein as the “Equity Line” or the “Oasis Equity Line.”
For more information about the selling stockholder, please see the section of this prospectus entitled “Selling Stockholder” beginning on page 28.
The selling stockholder may sell any shares offered under this prospectus at fixed prices, prevailing market prices at the time of sale, at varying prices or negotiated prices.
Oasis Capital is an “underwriter” within the meaning of the Securities Act of 1933, as amended (the “Securities Act”), in connection with the resale of our common stock under the Equity Line, and any broker-dealers or agents that are involved in such resales may be deemed to be “underwriters” within the meaning of the Securities Act in connection therewith. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. For more information, please see the section of this prospectus titled “Plan of Distribution” beginning on page 29.
We will not receive any proceeds from the resale of shares of common stock by the selling stockholder. We will, however, receive proceeds from the sale of shares directly to Oasis Capital pursuant to the Equity Line.
Our common stock is quoted on the OTCQB market operated by the OTC Markets Group, Inc., or “OTCQB,” under the ticker symbol “PPCB.” On February 15, 2019, the last reported sale price of our common stock was $0.015 per share.
Investing in our common stock involves risks that are described in the “Risk Factors” section beginning on page 6 of this prospectus.
You should rely only on the information contained in this prospectus or any prospectus supplement or amendment thereto. We have not authorized anyone to provide you with different information. This prospectus may only be used where it is legal to sell these securities. The information in this prospectus is only accurate on the date of this prospectus, regardless of the time of any sale of securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is 2019.
You should rely only on the information contained in this prospectus and any prospectus supplement prepared by or on behalf of us or to which we have referred you. We have not authorized anyone to provide you with information that is different. If anyone provides you with different or inconsistent information, you should not rely upon it. This prospectus is not an offer to sell, nor is the selling stockholder seeking an offer to buy, securities in any state where such offer or solicitation is not permitted. The information in this prospectus is complete and accurate only as of the date on the front cover of this prospectus, regardless of the time of delivery of this prospectus or any sale of shares of our common stock. Our business, financial condition, results of operations and prospects may have changed since that date.
Table of Contents
You should rely only on the information contained in this prospectus or in any free writing prospectus we may authorize to be delivered or made available to you. We have not authorized anyone to provide you with different information. We are offering to sell, and seeking offers to buy, shares of common stock only in jurisdictions where offers and sales are permitted. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of shares of our common stock. Our business, financial condition, operating results and prospects may have changed since that date.
Propanc Biopharma, Inc., the Propanc Biopharma logo, and other trademarks or service marks of Propanc Biopharma appearing in this prospectus are the property of Propanc Biopharma, Inc. This prospectus also includes trademarks, tradenames and service marks that are the property of other organizations. Solely for convenience, trademarks and tradenames referred to in this prospectus appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights, or that the applicable owner will not assert its rights, to these trademarks and tradenames.
| 2 |
The following summary highlights information contained elsewhere in this prospectus and does not contain all of the information that you should consider in making your investment decision in our common stock. Before investing in our common stock, you should carefully read this entire prospectus, including our consolidated financial statements and the related notes included in this prospectus and the information set forth under the headings “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
As used in this prospectus, unless the context otherwise requires, references to “we,” “us,” “our,” “Company,” “Propanc” refer to Propanc Biopharma, Inc. and our wholly owned subsidiary Propanc PPY LTD.
Our Business
We are a development-stage healthcare company that is currently focused on developing new cancer treatments for patients suffering from pancreatic, ovarian and colorectal cancer. Utilizing our scientific and oncology consultants, we have developed a rational, composite formulation of anti-cancer compounds, which together exert a number of effects designed to control or prevent tumors from recurring and spreading through the body. Our lead product candidate, PRP, is a variation upon our novel formulation and involves pro-enzymes, the inactive precursors of enzymes. As a result of positive early indications of the anti-cancer effects of our technology, over the last year we have conducted successful pre-clinical studies on PRP and subject to us receiving adequate financing, we hope to submit a clinical trial application in the 2019 calendar year. We intend to develop our PRP to treat early-stage cancer and pre-cancerous diseases and as a preventative measure for patients at risk of developing cancer based on genetic screening.
The Problem
In the early phases of tumor progression, cancer cells multiply near the site where their predecessors first began uncontrolled proliferation. The result, usually over a long period of time, is a primary tumor mass. Tumors often need to reach a large size before they make themselves apparent to the individual concerned, or the clinician screening for them.
Eventually, tumors of substantial size may begin to compromise the functioning of organs in which they have arisen and begin to evoke symptoms. In many cases, the effects on normal tissue function come from the physical pressure exerted by the expanding tumor masses. For example, large tumors in the colon may obstruct digestion products through the lumen, or in the lungs, airways may be compromised.
As dangerous and threatening as these primary tumors are, they are ultimately responsible for only about 10% of deaths. A far greater threat often arises for the patient, even after a primary tumor has been identified and removed. This threat involves cancerous growths that are discovered at sites far removed from the locations in their bodies where their primary tumors first appeared. These cancerous growths, called metastases, are responsible for approximately 90% of patient deaths from cancer. Metastases are formed by cancer cells that have left the primary tumor mass and traveled by the body’s blood and lymphatic vessels (a vein-like vessel carrying lymph, or white blood cells, from the tissues) to seek new sites and form new colonies. For example, breast cancers often spawn metastatic colonies in many tissues throughout the body including the brain, liver, bones, and lungs.
For primary tumors that have not yet metastasized, current treatments for cancer can be effective in initially reducing tumor burden. However, for many forms of cancer, current treatments lack sufficient efficacy to achieve a long lasting clinical response. Therefore, a vast majority of patients who succumb to cancer are killed by tumors that have metastasized. According to the National Cancer Institute’s SEER Cancer Statistics Review (2001 – 2007), of the patients diagnosed with late stage metastatic breast cancer, only 23% are expected to live longer than five years. This is compared to a 98% five-year survival rate for an early stage breast cancer patient when the cancer is confined to the primary site.
Our Solution
Our solution is to develop and commercialize a long-term therapy to prevent tumor recurrence and metastases, the main cause of patient death from cancer. We believe this problem can be addressed by developing a pro-enzyme formulation specifically targeting malignant carcinoma cells to create a long lasting clinical benefit to the patient.
Our lead product, PRP, is a novel, patented formulation consisting of two pro-enzymes, trypsinogen and chymotrypsinogen, combined at a ratio of one-to-six (1:6), to be administered intravenously. After establishing proof of concept in vivo as described in more detail below in the section captioned “Business”, supplemented by laboratory research at the Universities of Jaén and Granada on the mechanism of action of the pro-enzyme mixture, evidence suggests PRP may be effective against a range of solid tumors.
| 3 |
PRP recently completed preclinical development. A First-In-Human (FIH), Phase Ib study in patients with advanced solid tumors, evaluating the safety, pharmacokinetics and anti-tumor efficacy of PRP is planned to commence in 2019 subject to us receiving sufficient financing, and is hoped to be completed within twelve months. The study will be an open-label, multicenter, non-comparative study of PRP administered at increasing dose levels, with once daily intravenous injections over a 28-day cycle, with at least 20 and up to 40 patients enrolled.
The Phase Ib study is planned to be followed by two open Phase IIa studies evaluating the safety, pharmacokinetics and anti-tumor efficacy of PRP administered intravenously to patients with locally advanced or metastatic pancreatic adenocarcinoma, or to patients with advanced epithelial ovarian cancer who have failed prior anti-cancer therapy regimen. These studies are envisioned to start in parallel, shortly after the FIH Phase IIa study, and are hoped to commence in 2021. Both studies will be open, multicenter phase II studies measuring overall survival of patients having received once daily intravenous administrations of PRP.
Our Development Strategy
Our goal is to undertake early stage clinical development of PRP through to a significant value inflection point, where the commercial attractiveness of a drug in development, together with a greater likelihood of achieving market authorization, may attract potential interest from licensees seeking to acquire new products. Such value inflection points in the context of cancer drugs are typically at the point where formal, controlled clinical trials have demonstrated either ‘efficacy’ or ‘proof of concept’ – typically meaning that there is controlled clinical trial evidence that the drug is effective in the proposed target patient population, has an acceptable safety profile, and is suitable for further development. From a ‘big picture’ perspective, it is our intention to progress the development of our technology through the completion of our planned Phase IIa clinical trials and then to seek a licensee for further development beyond that point.
As part of that commercial strategy, we will:
| ● | continue research and development to build our existing intellectual property portfolio, and to seek new, patentable discoveries; | |
| ● | seek to ensure all product development is undertaken in a manner that makes our products approvable in the major pharmaceutical markets, including the U.S., Europe, the UK, Australia and Japan; | |
| ● | aggressively pursue the protection of our technology through all means possible, including patents in all major jurisdictions, and potentially trade secrets; and | |
| ● | make strategic acquisitions to acquire new companies that have products or services that complement our future goals. |
Our Development Plan and Milestones
We plan to progress PRP down a conventional early stage clinical development pathway for:
| ● | Regulatory and/or ethics approval to conduct a Phase Ib study and submit it with the applicable government agency for approval; and | |
| ● | Phase IIa multiple escalating dose studies to investigate the safety, tolerability, and pharmacokinetics of PRP administered intravenously to patients. |
We are currently evaluating Australia, UK and Europe as the potential destination where we may commence the Phase Ib trial. In particular, we are closely evaluating Australia because of its research and development tax incentives, as well as a simplified regulatory environment. As part of such incentives, eligible companies conducting clinical trials in Australia may receive up to 43.5% “cash-back” benefit in the form of a refund of their qualified research and development costs and expenses. We are evaluating all options to conduct our planned clinical trials in the most cost-efficient manner, while striving to minimize dilution to our stockholders.
We anticipate reaching the Phase IIa proof of concept milestone in approximately three to four years, subject to regulatory approval, and the results from our research and development and licensing activities.
Our overhead and expenses are likely to increase from their current level as PRP progresses down the development pathway. This increase will be driven by the need to increase our internal resources in order to effectively manage our research and development activities.
In the 2019 calendar year, we intend to initiate a Phase Ib study in advanced cancer patients with solid tumors and the anticipated costs will be approximately $2,500,000. We intend to use the proceeds of the Equity Line and/or additional financing to fund our planned Phase I, II and III clinical trials and for working capital.
Our principal executive office is located at 302, 6 Butler Street, Camberwell, VIC, 3124 Australia. Our telephone number is +61-03-9882-6723 and our website is www.propanc.com. Unless expressly noted, none of the information on our website is part of this prospectus or any prospectus supplement. Our common stock is quoted on the OTCQB market operated by the OTC Markets Group, Inc., or “OTCQB,” under the ticker symbol “PPCB.”
| 4 |
Offering SUMMARY
| Common stock that may be offered by selling stockholder | 111,203,954 shares | |
| Common stock outstanding before this offering | 333,896,577 shares (1) | |
| Common stock to be outstanding after this offering | 445,100,531 shares (2) | |
| Use of proceeds | We will not receive any proceeds from the resale or other disposition of the shares covered by this prospectus by the selling stockholder. We will receive proceeds from the sale of shares to Oasis Capital. Oasis Capital has committed to purchase up to $10,000,000 worth of shares of our common stock (the “Put Shares”) over a period of time terminating on the earlier of the date on which Oasis Capital shall have purchased shares under the Equity Purchase Agreement for an aggregate purchase price of $10,000,000 or February 25, 2022. | |
| Oasis Capital will pay a purchase price equal to 87.5% of the “Market Price,” which is defined as the one lowest daily volume weighted average traded price on the OTCQB, as reported by Bloomberg Finance L.P. or Quotestream, during the five trading days immediately following the date Oasis Capital receives the Put Shares via deposit/withdrawal at custodian share transfer method (“DWAC”) associated with the applicable put notice (the “Pricing Period”). In order to exercise the put, certain conditions must be met at each put notice date including, but not limited to: (i) we must have an effective registration statement covering the shares of our common stock that Oasis Capital has agreed to purchase from us pursuant as part of the Equity Line, (ii) our common stock must DWAC eligible, (iii) the minimum price must exceed $0.0001, and (iv) the number of shares to be purchased by Oasis Capital may not exceed the number of shares that, when added to the number of shares of our common stock then beneficially owned by Oasis Capital, would exceed 9.99% of our shares of common stock outstanding. | ||
| For further information, see “The Offering” beginning on page 26. | ||
| Plan of Distribution | The selling stockholder may, from time to time, sell any or all of their shares of our common stock on the stock exchange, market or trading facility on which the shares are traded or in private transactions. These sales may be at fixed or negotiated prices. | |
| For further information, see “Plan of Distribution” beginning on page 29. | ||
| Risk factors | You should read the “Risk Factors” section of this prospectus and the other information in this prospectus for a discussion of factors to consider carefully before deciding to invest in shares of our common stock. |
| (1) | The number of shares of our common stock issued and outstanding as of February 14, 2019. |
| (2) | Assumes the issuance of 111,203,954 shares offered hereby that are issuable under the Equity Purchase Agreement with Oasis Capital. |
| 5 |
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below, as well as the other information in this prospectus, including our consolidated financial statements and the related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” before deciding whether to invest in our common stock. The occurrence of any of the events or developments described below could harm our business, financial condition, operating results, and growth prospects. In such an event, the market price of our common stock could decline, and you may lose all or part of your investment. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may impair our business operations.
RISKS RELATED TO OUR FINANCIAL CONDITION AND OUR NEED FOR ADDITIONAL CAPITAL
Our independent registered public accounting firm has expressed concerns about our ability to continue as a going concern. Our ability to continue as a going concern is in substantial doubt absent obtaining adequate new debt or equity financings.
The report of our independent registered public accounting firm expresses concern about our ability to continue as a going concern based on the absence of revenues, recurring losses from operations and our need for additional financing to fund all of our operations. Working capital limitations continue to impinge on our day-to-day operations, thus contributing to continued operating losses. For the six months ended December 31, 2018 and June 30, 2018, we had net losses of $3,574,974 and $4,125,722, respectively. For the fiscal years ended June 30, 2018 and June 30, 2017, we had net losses of $7,039,155 and $7,867,500, respectively. Further, as of December 31, 2018, we had $124,538 in cash and $8,639 in receivable accounts and had an accumulated deficit of $48,857,652.
Based upon our current business plan, we will need considerable cash investments to be successful. Our capital requirements and cash needs are significant and continuing. We can provide no assurance that we will be able to generate a sufficient amount of revenue, if any, from our business in order to achieve profitability. It is not possible at this time for us to predict with assurance the potential success of our business. The revenue and income potential of our proposed business and operations are unknown. If we cannot continue as a viable entity, we may be unable to continue our operations and you may lose some or all of your investment in our common stock.
We have incurred significant losses since our inception. We expect to incur significant losses for the foreseeable future and may never achieve or maintain profitability.
Since inception, we have incurred significant operating losses. Our net loss was $3,574,974 and $4,125,722, respectively, for the six months ended December 31, 2018 and December 31, 2017. As of December 31, 2018, we had accumulated deficit of $48,857,652. Our net loss was $7,039,155 and $7,867,500, respectively, for the fiscal years ended June 30, 2018 and June 30, 2017. As of June 30, 2018, we had accumulated deficit of $45,282,678. To date, we have not generated any revenues and have financed most of our operations with funds obtained from private financings.
Since October 2007, we have devoted substantially all of our efforts to research and development of our product candidates, particularly PRP, and efforts to protect our intellectual property. Most recently, from January-February 2016, and October 2016-April 2017, we have contracted with third parties to perform a number of laboratory studies and dose range finding studies designed to examine the anti-cancer effects of PRP and prepare for human clinical trials. Since mid-2017, we developed a suitable manufacturing process for each active drug substance in the PRP formulation, capable of producing a full scale GMP manufacture of PRP for human trials. We were granted Orphan Drug Designation status from the FDA for PRP for the treatment of pancreatic cancer. In March 2018, a scientific advice meeting was conducted with the MHRA (Medicines and Healthcare Products Regulatory Agency) UK, to assist with preparation of our first Clinical Trial Application (CTA). We expect that it will be many years, if ever, before we have a product candidate ready for commercialization. We expect to incur significant expenses and increasing operating losses for the foreseeable future if and as we progress PRP into clinical trials, continue our research and development, seek regulatory approvals, establish a sales and marketing infrastructure, maintain and expand our intellectual property portfolio, and add personnel.
To become profitable, we must develop and eventually commercialize PRP, or some other product with significant market potential. This will require us to successfully complete clinical trials, obtain market approval and market and sell PRP or whatever other product that we obtain approval for. We might not succeed in any one or a number of these activities, and even if we do, we may never generate revenues that are significant enough to achieve profitability. Our failure to become and remain profitable would decrease our value and could impair our ability to raise capital, maintain our research and development efforts, expand our business or continue our operations.
| 6 |
As an early stage company, it may be difficult for you to evaluate the success of our business to date and to assess our future viability.
Despite having been founded in 2007, we remain an early-stage company. We commenced active operations in the second half of 2010. Our operations to date have been mainly limited to establishing our research programs, particularly PRP, building our intellectual property portfolio and deepening our scientific understanding of our product development. We have not yet initiated, let alone demonstrated any ability to successfully complete, any clinical trials, including large-scale, pivotal clinical trials, obtain marketing approvals, manufacture a commercial scale product, or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. It will take a number of years for PRP to be made available for the treatment of cancer, if it ever is. Given our relatively short operating history compared to the timeline required to fully develop a new drug, you are cautioned about making any predictions on our future success or viability based on our activities or results to date. In addition, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors. We will eventually need to transition from a company with a research focus to a company capable of supporting commercial activities. We may not be successful in such a transition.
We currently rely, and may continue to rely for the foreseeable future, on substantial debt financing that we are not able to repay in cash.
In order to maintain our operations, including our research and development efforts and our preclinical development of PRP, we have over the last three years entered into a number of securities purchase agreements pursuant to which we issued convertible debt in return for cash. We are not currently able to repay either the current principal or interest on this debt in cash. Our lenders, therefore, can convert their debt into shares of our common stock, at a percentage discount to current market prices and then attempt to sell these shares on the open market in order to pay down their loans and receive a return on their investment. These financings pose the risk that as these debts are converted, our stock price will reflect the reduced prices our lenders are willing to sell their shares at, given the discount they have received. These financings contain no floor on the price our lenders can convert their debt into shares of our common stock and they could conceivably reduce the price our common stock to near zero. These types of financings negatively impact our balance sheet and the appeal of our common stock as an investment. While we are actively exploring various alternatives to reduce if not eliminate this debt, for the foreseeable future we will continue to carry it on our balance sheet, and we may have to enter into additional such financings in order to sustain our operations. As a result, the price of our common stock and our market capitalization are subject to significant declines until our convertible debt is either refinanced on a favorable basis or is eliminated.
As of December 31, 2018, the total amount of debt outstanding under these convertible notes, including interest, is $1,756,091 (not including redemption premium). Please see the section captioned “Management’s Discussion of Financial Condition and Results of Operations - Recent Developments” for further information.
We will continue to need substantial additional funding. If we are unable to raise capital when needed, we would be forced to delay, reduce or eliminate our product development programs or commercialization efforts.
We expect our expenses to significantly increase in connection with our ongoing activities, particularly if we initiate clinical trials of, and ultimately seek marketing approval for, PRP. In addition, even if we ultimately obtain marketing approval for PRP or any other product candidate, we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing and distribution. We also hope to continue and expand our research and development activities. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we would be forced to delay, reduce or eliminate our future commercialization efforts or any research and development programs.
Our future capital requirements will depend on many factors, including, among others, the scope, progress and, results of our potential future clinical trials, the costs, timing and outcome of regulatory review of PRP, the costs of any future commercialization activities, and the costs of preparing and filing future patent applications, if any. Accordingly, we will continue to rely on additional financing to achieve our business objectives. Adequate additional financing, may not be available to us on acceptable terms, or at all. Even if we are able to enter into financing agreements, we may be forced to pay higher interest rates, accept default provisions in financing agreements that we believe are overly punitive, make balloon payments as required, and, as noted below, if we issue convertible debt the price of our common stock may well be negatively affected and our existing shareholders may suffer dilution.
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial product revenues, we expect to continue to finance our cash needs through a combination of equity offerings and additional debt financings, and possibly also through future collaborations, strategic alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or debt securities, including convertible debt securities, the ownership interest of our existing stockholders will be diluted upon conversion, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our existing stockholders.
| 7 |
Debt financing, if available, may also involve agreements that include restrictive covenants limiting or restricting our ability to take specific actions, such as merging with other companies or consummating certain changes of control, acquiring other companies, engaging in new lines of business, incurring additional debt, making capital expenditures, making certain investments, paying dividends, transferring or disposing of assets, amending certain material agreements, incurring additional indebtedness or enter into various specified transactions. We therefore may not be able to engage in any of the foregoing transactions unless we obtain the consent of the lender or terminate such debt agreements. Our debt agreements may also contain certain financial covenants, including achieving certain milestones and may be secured by substantially all of our assets. In the event we enter into such debt agreements, there is no guarantee that we will be able to generate sufficient cash flow or sales to pay the principal and interest under our debt agreements or to satisfy all of the financial covenants.
If we raise additional funds through collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
The conversion of some or all of our currently outstanding convertible notes in shares of our common stock will dilute the ownership interests of existing stockholders.
The conversion of some or all of our currently outstanding convertible notes in shares of our common stock will dilute the ownership interests of existing stockholders. As of December 31, 2018, we had 14 outstanding notes convertible into up to 215,153,929 shares of our common stock (based on then applicable conversion prices). Each holder of the notes has agreed to a 4.99% beneficial ownership conversion limitation (subject to certain noteholders’ ability to increase such limitation to 9.99% upon 60 days’ notice to us), and each note may not be converted during the first six-month period from the date of issuance. Any sales in the public market of the common stock issuable upon such conversion or any anticipated conversion of our convertible notes into shares of our common stock could adversely affect prevailing market prices of our common stock.
The accounting method for convertible debt securities that may be settled in cash could have a material adverse effect on our reported financial results.
Under Financial Accounting Standards Board Accounting Standards Codification 470-20, Debt with Conversion and Other Options (“ASC 470-20”), we are required to separately account for the liability and equity components of our convertible notes because they may be settled entirely or partially in cash upon conversion in a manner that reflects our economic interest cost. The effect of ASC 470-20 on the accounting for our convertible notes is that the equity component is required to be included in the additional paid-in capital section of stockholders’ deficit on our consolidated balance sheet, and the value of the equity component would be treated as original issue discount for purposes of accounting for the debt component of our convertible notes. As a result, we will be required to record a greater amount of non-cash interest expense in current periods presented as a result of the amortization of the discounted carrying value of our convertible debt or notes to their face amount over the terms. We will report higher net loss in our financial results in part because ASC 470-20 will require interest to include both the current period’s amortization of the debt discount and the instrument’s coupon interest, which could adversely affect our reported or future financial results, the trading price of our common stock and the trading price of our convertible notes.
In addition, because our convertible notes may be settled entirely or partly in cash, under certain circumstances, these are currently accounted for utilizing the treasury stock method, the effect of which is that the shares issuable upon conversion are not included in the calculation of diluted earnings per share except to the extent that the conversion value exceeds their principal amount. Under the treasury stock method, for diluted earnings per share purposes, the transaction is accounted for as if the number of shares of common stock that would be necessary to settle such excess, if we elected to settle such excess in shares, are issued. We cannot be sure that the accounting standards in the future will continue to permit the use of the treasury stock method. If we are unable to use the treasury stock method in accounting for the shares issuable upon conversion of our convertible notes, then our diluted earnings per share would be adversely affected.
We maintain our cash in Australian financial institutions that are not insured.
The Company maintains its cash in banks and financial institutions in Australia. Bank deposits in Australian banks are uninsured. The Company has not experienced any losses in such accounts through the date of the filing of this registration statement of which this prospectus is a part.
| 8 |
RISKS RELATED TO THE DISCOVERY, DEVELOPMENT AND COMMERCIALIZATION OF OUR PRODUCT CANDIDATES
Because PRP remains in the early stages of development and may never become commercially viable, you may lose your investment.
At present, our lead product candidate, PRP, is still in preclinical development. While we are hopeful that the preclinical testing we have completed will lead to our initiating human clinical trials in 2019, as noted elsewhere we expect that it will be several years, at least, before PRP can be commercialized. Further, if clinical trials for PRP fail to produce statistically significant results, we would likely be forced to either spend several more years in development attempting to correct whatever flaws were identified in the trials, or we would have to abandon PRP altogether. Either of those contingencies, and especially the latter, would dramatically increase the amount of time before we would be able to generate any product-related revenue, and we may well be forced to cease operations. Under such circumstances, you may lose at least a portion of, and perhaps your entire, investment.
PRP may cause undesirable side effects that could negatively impact its clinical trial results or limit its use, hindering further development, subject us to possible product liability claims, and make it more difficult to commercialize PRP.
In addition to the possibility that the clinical trials we hope to initiate for PRP could demonstrate a lack of efficacy, if we alternatively identify adverse and undesirable side effects caused by it this will likely interrupt, delay or even halt our further development, or possibly limit our planned therapeutic uses for it, and may even result in adverse regulatory action by the FDA or other regulatory authorities.
Moreover, this may subject us to product liability claims by the individuals enrolled in our clinical trials; while we intend to obtain product liability insurance in connection with our clinical trials, it is possible that the potential liability of any claims against us could exceed the maximum amount of this coverage, or at least increase our premiums. Either would result in an increase in our operating expenses, in turn making it more difficult to complete our clinical development, or in the suspension or termination of the clinical trial. Any negative information concerning PRP, however unrelated to its composition or method of use, could also damage our chances to obtain regulatory approval.
Even if we are able to complete PRP’s development and receive regulatory approvals, undesirable side effects could prevent us from achieving or maintaining market acceptance of the product or substantially increase the costs and expenses of commercializing it.
Because successful development of our products is uncertain, our results of operations may be materially harmed.
Our development of PRP and future product candidates is subject to the risks of failure inherent in the development of new pharmaceutical products that are based on new technologies, including but not limited to delays in product development, clinical testing or manufacturing; unplanned and higher expenditures; adverse findings relating to safety or efficacy; failure to receive regulatory approvals; the emergence of superior or equivalent products; an inability by us or one of our collaborators to manufacture our product candidates on a commercial scale on our own, or in collaboration with third parties; and, ultimately, a failure to achieve market acceptance.
Because of these risks, our development efforts may not result in PRP, or any other product we attempt to develop, becoming commercially viable. If even one aspect of these development efforts is not successfully completed, required regulatory approvals will not be obtained, or if any approved products are not commercialized successfully, our business, financial condition and results of operations will be materially harmed.
A variety of factors, either alone or in concert with each other, could result in our clinical trials of PRP being delayed or unsuccessful.
While we have conducted a variety of preclinical studies, which we have concluded provide evidence to support the potential therapeutic utility of PRP, comprehensive clinical trials in order to demonstrate the product’s safety, tolerability and efficacy will now need to be completed. Clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. A failure of one or more clinical trials can occur at any stage of testing. The outcome of preclinical testing and even early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their products.
| 9 |
Among the numerous unforeseen events that may occur during, or as a result of, clinical trials that alone or in concert with each other could either delay or prevent our ability to receive marketing approval or commercialize PRP are the following:
| ● | regulators or institutional review boards may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site; | |
| ● | we may have delays in reaching or fail to reach an agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites; | |
| ● | as noted previously, clinical trials of PRP may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical trials or abandon product development altogether; | |
| ● | the number of patients required for clinical trials may be larger than we anticipate, enrollment in these clinical trials may be slower than we anticipate or participants may drop out of these clinical trials at a higher rate than we anticipate; | |
| ● | our third-party contractors may fail to comply with regulatory requirements or fail to meet their contractual obligations to us in a timely manner, or at all; |
| ● | regulators or institutional review boards may require that we or our investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks; | |
| ● | the cost of clinical trials may be greater than we anticipate; | |
| ● | the supply or quality of PRP or other materials necessary to conduct its clinical trials may be insufficient or inadequate; and | |
| ● | PRP may, as also noted above, have undesirable side effects or other unexpected characteristics, causing us or our investigators, regulators or institutional review boards to suspend or terminate the trials. |
If we are required to conduct additional clinical trials or other testing of PRP beyond those that we currently contemplate, if we are unable to successfully complete clinical trials of PRP or other testing, if the results of these trials or tests are not positive or are only modestly positive or if there are safety concerns, we may:
| ● | be delayed in obtaining marketing approval; | |
| ● | not obtain marketing approval at all; | |
| ● | obtain approval for indications or patient populations that are not as broad as intended or desired; | |
| ● | obtain approval with labeling that includes significant use or distribution restrictions or safety warnings, including boxed warnings; | |
| ● | be subject to additional post-marketing testing requirements; or | |
| ● | fail to obtain that degree of market acceptance necessary for commercial success. |
Any delay in, or termination of, our clinical trials may result in increased development costs, which would very likely cause the market price of our shares to decline and severely limit our ability to obtain additional financing and, ultimately, our ability to commercialize our products and generate product revenues. This in turn would likely materially harm our business, financial condition and operating results, and possibly lead us to cease operations.
If we fail to obtain regulatory approval in jurisdictions outside the United States, we will not be able to market PRP in those jurisdictions.
We intend to seek regulatory approval for PRP in the United Kingdom, Europe, Australia and/or other countries outside of the United States and expect that these countries will be important markets for our products, if approved. Marketing our products in these countries will require separate regulatory approvals in each market and compliance with numerous and varying regulatory requirements. The regulations that apply to the conduct of clinical trials and approval procedures vary from country to country and may require additional testing. Moreover, the time required to obtain approval may differ from that required to obtain FDA approval.
If, in the future, we are unable to establish sales and marketing capabilities or enter into agreements with third parties to sell and market PRP, we may not be successful in commercializing our product candidates if and when they are approved.
We do not have a sales or marketing infrastructure and have no experience in the sale, marketing or distribution of pharmaceutical products. To achieve commercial success for PRP or any other approved product, we must either develop a sales and marketing organization or outsource these functions to third parties. In the future, we may choose to build a focused sales and marketing infrastructure to market or co-promote some of our product candidates if and when they are approved.
| 10 |
There are risks involved with both establishing our own sales and marketing capabilities and entering into arrangements with third parties to perform these services. For example, recruiting and training a sales force is expensive and time consuming and could delay any product launch. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
Factors that may inhibit our efforts to commercialize our products on our own include:
| ● | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; | |
| ● | the inability of sales personnel to obtain access to physicians or persuade an adequate numbers of physicians to prescribe any future products; | |
| ● | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and | |
| ● | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
If we enter into arrangements with third parties to perform sales, marketing and distribution services, our product revenues or the profitability of these product revenues to us are likely to be lower than if we were to market and sell any products that we develop ourselves. In addition, we may not be successful in entering into arrangements with third parties to sell and market our product candidates or may be unable to do so on terms that are favorable to us. We likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively. If we do not establish sales and marketing capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing PRP.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of new drug products is highly competitive. We face competition with respect to our current product candidates, and will face competition with respect to any product candidates that we may seek to develop or commercialize in the future from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. There are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of products for the treatment of the disease indications for which we are developing our product candidates. Some of these competitive products and therapies are based on scientific approaches that are the same as or similar to our approach, and others are based on entirely different approaches. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
We are developing PRP for the treatment of pancreatic, ovarian and colorectal cancer. There are a variety of available therapies marketed for cancer. In many cases, these drugs are administered in combination to enhance efficacy. Some of these drugs are branded and subject to patent protection, and others are available on a generic basis. Many of these approved drugs are well-established therapies and are widely accepted by physicians, patients and third-party payors. Insurers and other third-party payors may also encourage the use of generic products. We expect that if our product candidates are approved, they will be priced at a significant premium over competitive generic products. This may make it difficult for us to achieve our business strategy of using PRP in combination with existing therapies or replacing existing therapies with PRP.
There are also a number of products in clinical development by other parties to treat and prevent metastatic cancer. Our competitors may develop products that are more effective, safer, more convenient or less costly than any that we are developing or that would render our product candidates obsolete or non-competitive. In addition, our competitors may discover biomarkers that more efficiently measure their effectiveness to treat and prevent metastatic cancer, which may give them a competitive advantage in developing potential products. Our competitors may also obtain marketing approval from the FDA or other regulatory authorities for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market.
Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. In addition, to the extent that product or product candidates of our competitors demonstrate serious adverse side effects or are determined to be ineffective in clinical trials, the development of our product candidates could be negatively impacted.
| 11 |
Even if we are able to commercialize PRP, we will need to seek approval for reimbursement before it can be marketed, and it may become subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, which would harm our business.
The regulations that govern marketing approvals, pricing and reimbursement for new drug products vary widely from country to country. In the United States, recently passed legislation may significantly change the approval requirements in ways that could involve additional costs and cause delays in obtaining approvals. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we might obtain marketing approval for PRP in a particular country, but then be subject to price regulations that delay our commercial launch of it, possibly for lengthy time periods, and negatively impact the revenues we are able to generate from the sale of PRP in that country. Adverse pricing limitations may hinder our ability to recoup our investment in PRP, even after it has obtained marketing approval.
Our ability to commercialize PRP successfully also will depend in part on the extent to which reimbursement for it will be available from government health administration authorities, private health insurers and other organizations. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. We cannot be sure that reimbursement will be available for PRP that we commercialize and, if reimbursement is available, the level of reimbursement. Reimbursement may impact the demand for, or the price of, PRP. Obtaining reimbursement for it may be particularly difficult because of the higher prices often associated with drugs administered under the supervision of a physician. If reimbursement is not available or is available only to limited levels, we may not be able to successfully commercialize PRP.
There may be significant delays in obtaining reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or similar regulatory authorities outside the United States. Moreover, eligibility for reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. Our inability to promptly obtain coverage and profitable payment rates from both government-funded and private payors for any approved products that we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
RISKS RELATED TO OUR DEPENDENCE ON THIRD PARTIES
We may depend on collaborations with third parties for the development and commercialization of PRP and other product candidates, and these collaborations may be unsuccessful.
We currently seek third-party collaborators for the development and commercialization of PRP, contract manufacturers (CMOs), contract research organizations (CROs), regulatory and development consultants, and hospitals for clinical trial sites. We intend to continue to rely on third-party collaborators for current and future product candidates for the foreseeable future. Our likely collaborators for any collaboration arrangements include large and mid-size pharmaceutical companies, regional and national pharmaceutical companies and biotechnology companies. If we do enter into any such arrangements with any third parties, we will likely have limited control over the amount and timing of resources that our collaborators dedicate to the development or commercialization of our product candidates. Our ability to generate revenues from these arrangements will depend on our collaborators’ abilities to successfully perform the functions assigned to them in these arrangements.
| 12 |
Collaborations involving our product candidates would pose the following risks to us:
| ● | collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations; | |
| ● | collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in the collaborator’s strategic focus or available funding or external factors such as an acquisition that diverts resources or creates competing priorities; | |
| ● | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; | |
| ● | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours; |
| ● | collaborators with marketing and distribution rights to one or more products may not commit sufficient resources to the marketing and distribution of such product or products; | |
| ● | collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our proprietary information or expose us to potential litigation; | |
| ● | disputes may arise between the collaborators and us that result in the delay or termination of the research, development or commercialization of our products or product candidates or that result in costly litigation or arbitration that diverts management attention and resources; and | |
| ● | collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates. |
Collaboration agreements may not lead to development or commercialization of product candidates in the most efficient manner or at all. If a present or future collaborator of ours were to be involved in a business combination, the continued pursuit and emphasis on our product development or commercialization program could be delayed, diminished or terminated.
If we are not able to establish collaborations, we may have to alter our development and commercialization plans.
Our potential commercialization of PRP will require substantial additional cash to fund clinical trial and other expenses. As noted above, we may decide to collaborate with other pharmaceutical and biotechnology companies for the development and potential commercialization of PRP and perhaps future product candidates as well.
We face significant competition in seeking appropriate collaborators. Whether we reach a definitive agreement for collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of clinical trials, the likelihood of approval by the FDA or similar regulatory authorities outside the United States, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on and whether such collaboration could be more attractive than the one with us for our product candidate. We may also be restricted under existing license agreements from entering into agreements on certain terms with potential collaborators. Collaborations are complex and time-consuming to negotiate and document. In addition, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators.
We may not be able to negotiate collaborations on a timely basis, on acceptable terms, or at all. If we are unable to do so, we may have to curtail the development of such product candidate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms or at all. If we do not have sufficient funds, we may not be able to further develop our product candidates or bring them to market and generate product revenue.
| 13 |
We currently contract with a third party for the manufacture of PRP and this third party may not perform satisfactorily or at all, and our reliance on any third-party for the supply of PRP carries material risks.
We do not have any manufacturing facilities or personnel. We currently obtain all of our supply of PRP for clinical development through our Manufacturing Service Agreement (the “MSA”) with Amatsigroup, and we expect to continue to rely on Amatsigroup for the manufacture of clinical and, if necessary, commercial quantities of PRP. We anticipate that our payments to Amatsigroup under the MSA will range between $2.5 million and $5.0 million over three years, when the finished drug product is manufactured and released for clinical trials. We have spent a total of $1,689,146 of costs to date under the MSA of which $1,639,192 was expensed in the years ended June 30, 2018 and 2017 and $49,854 was expensed in the six months ended December 31, 2018. The MSA shall continue for a term of three years unless extended by mutual agreement in writing. Either party to the MSA has the right to terminate the MSA by written notice to the other party if the other party commits a material breach of the MSA (subject to a 30-day cure period). If we are not current with payments to Amatsigroup and Amatsigroup terminates the MSA or suspends its manufacturing services to us, this adversely affect our supply of PRP and result in harm to our business and results of operations.
This reliance on a third party increases the risk that we will not have sufficient quantities of PRP on hand at any given time, which could delay, prevent or impair our development efforts. We do not currently have alternative arrangements in place to supply us with PRP should Amatsigroup fail to perform for any reason. Amatsigroup may also fail to comply with current good manufacturing practices (“cGMP”) regulations or similar regulatory requirements outside the United States. Any such failure to comply with applicable regulations could result in sanctions being imposed on Amatsigroup, and possibly us as well. These sanctions could include fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of PRP, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect our supply of PRP and result in harm to our business and results of operations.
PRP and any other product that we may develop may compete with other product candidates and products for access to manufacturing facilities. Although we believe that there are several potential alternative manufacturers who could manufacture PRP, we may incur added costs and delays in identifying and qualifying any such replacement, as well as producing the drug product. In addition, we would then have to enter into technical transfer agreements and share our know-how with the new third-party manufacturers, which can be time-consuming and may result in delays.
Even if we were able to quickly establish agreements with other third-party manufacturers, our general reliance on third-party manufacturers entails many of the same risks as our agreement with Amatsigroup, including:
| ● | reliance on the third party for regulatory compliance and quality assurance; | |
| ● | the possible breach of the manufacturing agreement by the third party, including the misappropriation of our proprietary information, trade secrets and know-how; | |
| ● | the possible termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us; and | |
| ● | disruptions to the operations of our manufacturers or suppliers caused by conditions unrelated to our business or operations, including the bankruptcy of the manufacturer or supplier or a catastrophic event affecting our manufacturers or suppliers. |
Our current reliance on the services of Amatsigroup and current and anticipated future dependence upon others for the manufacture of PRP may adversely affect our future profit margins and our ability to commercialize any products that receive marketing approval on a timely and competitive basis.
RISKS RELATED TO OUR INTELLECTUAL PROPERTY
If we fail to comply with our obligations under any intellectual property licenses with third parties, we could lose license rights that are important to our business.
We are currently a party to a joint commercialization agreement with the University of Bath, and hope to enter into other license agreements in the future. If we fail to comply with the obligations included in any future license we may enter into in the future, such licensors may have the right to terminate these agreements, in which event we might not be able to market any product that is covered by the agreements, or to convert the exclusive licenses to non-exclusive licenses, which could materially adversely affect the value of the product candidate being developed under these license agreements. As a general matter, termination of license agreements or reduction or elimination of our licensed rights may result in our having to negotiate new or reinstated licenses with less favorable terms.
| 14 |
If we are unable to obtain and maintain patent protection for our technology and products, or if any licensors are unable to obtain and maintain patent protection for the technology or products that we may license from them in the future, or if the scope of the patent protection obtained is not sufficiently broad, our competitors could develop and commercialize technology and products similar or identical to ours, and our ability to successfully commercialize our technology and products may be adversely affected.
We have obtained patent protection for PRP in seven countries, and have a patent application either pending or under examination in eight others, including the United States and the European Union. Our future success depends in large part on our and, as applicable, our licensors’, ability to obtain and maintain patent protection in the United States and other countries with respect to our proprietary technology. We cannot be certain that patents will be issued in those countries where our applications are still under examination.
The patent process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are uncertain. Our pending and future patent applications may not result in patents being issued which protect our technology or products or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection.
The laws of foreign countries may not protect our rights to the same extent as the laws of the United States. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing. Therefore, we cannot be certain that we or our licensors were the first to make the inventions claimed in our owned or licensed patents or pending patent applications, or that we or our licensors were the first to file for patent protection of such inventions.
Assuming the other requirements for patentability are met, in the United States, for patents that have an effective filing date prior to March 15, 2013, the first to make the claimed invention is entitled to the patent, while outside the United States, the first to file a patent application is entitled to the patent. In March 2013, the United States transitioned to a first inventor to file system in which, assuming the other requirements for patentability are met, the first inventor to file a patent application will be entitled to the patent. We may be subject to a third party preissuance submission of prior art to the U.S. Patent and Trademark Office, or become involved in opposition, derivation, reexamination, inter parties review or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights.
Even if our owned and licensed patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
We may become involved in lawsuits to protect or enforce our patents, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our patents. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours is invalid or unenforceable or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, our licensors may have rights to file and prosecute such claims and we are reliant on them.
| 15 |
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.
Our commercial success depends upon our ability and the ability of our collaborators to develop, manufacture, market and sell PRP and any other product candidates and use our proprietary technologies without infringing the proprietary rights of third parties. We have yet to conduct comprehensive freedom-to-operate searches to determine whether our use of certain of the patent rights owned by or licensed to us would infringe patents issued to third parties. We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology, including interference proceedings before the U.S. Patent and Trademark Office and their European Union and global equivalents. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future. If we are found to infringe a third party’s intellectual property rights, we could be required to obtain a license from such third party to continue developing and marketing our products and technology. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, we could be found liable for monetary damages. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for some of our technology and products, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
RISKS RELATED TO REGULATORY APPROVAL OF OUR PRODUCT CANDIDATES AND OTHER LEGAL COMPLIANCE MATTERS
If we are not able to obtain, or if there are delays in obtaining, required regulatory approvals, we will not be able to commercialize PRP, and our ability to generate revenue will be materially impaired.
PRP and the activities associated with its development and commercialization, including design, testing, manufacture, safety, efficacy, recordkeeping, labeling, storage, approval, advertising, promotion, sale and distribution, are subject to comprehensive regulation by the FDA and other regulatory agencies in the United States and by comparable authorities in other countries. Failure to obtain marketing approval for PRP will prevent us from commercializing it. We have not received approval to market PRP or any other product candidate from regulatory authorities in any jurisdiction. We have only limited experience in filing and supporting the applications necessary to gain marketing approvals and expect to rely on third-party contract research organizations to assist us in this process. Securing FDA approval requires the submission of extensive preclinical and clinical data and supporting information to the FDA for each therapeutic indication to establish PRP’s safety and efficacy. Securing FDA approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the FDA. PRP may not be effective, may be only moderately effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude our obtaining marketing approval or prevent or limit commercial use.
| 16 |
The process of obtaining marketing approvals, both in the United States and abroad, is expensive, may take many years if additional clinical trials are required, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved. Changes in marketing approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application, may cause delays in the approval or rejection of an application. The FDA has substantial discretion in the approval process and may refuse to accept any application or may decide that our data is insufficient for approval and require additional preclinical, clinical or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent marketing approval of a product candidate. Any marketing approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
If we experience delays in obtaining approval or if we fail to obtain approval of PRP, the commercial prospects for PRP may be harmed and our ability to generate revenues will be materially impaired.
Failure to obtain marketing approval in international jurisdictions would prevent PRP from being marketed abroad.
We intend to seek regulatory approval for PRP in a number of countries outside of the United States and expect that these countries will be important markets for it, if approved. In order to market and sell our products in the European Union, the UK, Australia and many other jurisdictions, we or our third-party collaborators must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain FDA approval. The regulatory approval process outside the United States generally includes all of the risks associated with obtaining FDA approval. In addition, in many countries outside the United States, it is required that the product be approved for reimbursement before the product can be approved for sale in that country. We or these third parties may not obtain approvals from regulatory authorities outside the United States on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the United States does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our products in any market.
PRP or any other product candidate for which we obtain marketing approval could be subject to restrictions or withdrawal from the market and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our products, when and if any of them are approved.
PRP, or any other product candidate for which we obtain marketing approval, along with the manufacturing processes, post-approval clinical data, labeling, advertising and promotional activities for such product, will be subject to continual requirements of and review by the FDA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, registration and listing requirements, cGMP requirements relating to quality control, quality assurance and corresponding maintenance of records and documents, requirements regarding the distribution of samples to physicians and recordkeeping. Even if marketing approval of a product candidate is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the product. The FDA closely regulates the post-approval marketing and promotion of drugs to ensure drugs are marketed only for the approved indications and in accordance with the provisions of the approved labeling. The FDA imposes stringent restrictions on manufacturers’ communications regarding off-label use and if we do not market our products for their approved indications, we may be subject to enforcement action for off-label marketing.
In addition, later discovery of previously unknown problems with our products, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various results, including:
| ● | restrictions on such products, manufacturers or manufacturing processes; | |
| ● | restrictions on the labeling or marketing of a product; | |
| ● | restrictions on product distribution or use; | |
| ● | requirements to conduct post-marketing clinical trials; | |
| ● | warning or untitled letters; | |
| ● | withdrawal of the products from the market; | |
| ● | refusal to approve pending applications or supplements to approved applications that we submit; |
| 17 |
| ● | recall of products; | |
| ● | fines, restitution or disgorgement of profits or revenue; | |
| ● | suspension or withdrawal of marketing approvals; | |
| ● | refusal to permit the import or export of our products; | |
| ● | product seizure; or | |
| ● | injunctions or the imposition of civil or criminal penalties. |
Our current attempts to both expand our patent protection and seek regulatory approvals from multiple countries, as well as our future relationships with customers and third-party payors will be subject to applicable anti-kickback, fraud and abuse and other laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
As we seek to obtain patent protection from multiple jurisdictions and eventually to seek marketing approval for PRP in those counties, we are and will continue to be subject to the Foreign Corrupt Practices Act, which makes it illegal for any U.S. business, even one like Propanc that is physically located in another country, to influence foreign officials with personal payments and rewards.
Moreover, healthcare providers, physicians and third-party payors will play a primary role in the recommendation and prescription of PRP and any other product candidate for which we obtain marketing approval. Our future arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations, include the following:
| ● | the federal healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid; | |
| ● | the federal False Claims Act imposes criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; | |
| ● | the federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; | |
| ● | the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; | |
| ● | the federal transparency requirements under the Health Care Reform Law requires manufacturers of drugs, devices, biologics and medical supplies to report to the Department of Health and Human Services information related to physician payments and other transfers of value and physician ownership and investment interests; and | |
| ● | analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines and exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other providers or entities with whom we expect to do business are found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
| 18 |
Recently enacted and future legislation, particularly in the United States, may increase the difficulty and cost for us to obtain marketing approval of and commercialize PRP and affect the prices we may obtain.
In the United States and some foreign jurisdictions there have been many legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain marketing approval.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (“Medicare Modernization Act”), changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for physician-administered drugs. In addition, this legislation provided authority for limiting the number of drugs that will be covered in any therapeutic class. Cost reduction initiatives and other provisions of this legislation could decrease the coverage and price that we receive for any approved products. While the Medicare Modernization Act applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the Medicare Modernization Act may result in a similar reduction in payments from private payors.
In March 2010, President Obama signed into law the Patient Protection and Affordable Care Act (“Affordable Care Act”), a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for health care and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. Among other things, the Affordable Care Act revised the definition of “average manufacturer price” for reporting purposes, which could increase the amount of Medicaid drug rebates to states, and it imposed a significant annual fee on companies that manufacture or import branded prescription drug products.
At present, the future of the Affordable Care Act is the subject of significant debate in the U.S. Congress, with proposals to either partially or entirely repeal it being considered and the likelihood that there will be a new law to replace it is uncertain. It is not yet possible for us to determine the impact, if any, the enactment of any of these proposals will have on our future ability to obtain approval of or commercialize PRP.
The UK’s decision to leave the European Union could significantly increase regulatory burdens on obtaining approvals for PRP within the UK.
On March 29, 2017, the UK invoked Article 50 of Lisbon Treaty to initiate complete withdrawal from the European Union by March 30, 2019, and therefore, the regulatory drug approval process in that country may be significantly different from the current drug regulatory policies in the European Union. We currently are considering holding our clinical trials in the UK, among other countries, and therefore this event could significantly impact our efforts to successfully bring PRP to market. It is not yet possible for us to determine the impact of the UK’s withdrawal from the European Union, but any additional costs or delays in obtaining approvals may hinder our ability to conduct clinical trials or market PRP in the UK.
RISKS RELATING TO EMPLOYEE MATTERS AND MANAGING GROWTH
Our future success depends on our ability to retain our chief executive officer and our chief scientific officer and, as we continue to develop and grow as a company, to attract, retain and motivate qualified personnel.
We are highly dependent on our management team, specifically Mr. James Nathanielsz, our Chief Executive Officer, Chief Financial Officer, Acting Chairman, Secretary, Treasurer and a director, and Dr. Julian Kenyon, our director who also serves as our chief scientific officer in a non-executive officer capacity. While we have a current employment agreement with Mr. Nathanielsz and a director agreement with Dr. Kenyon, both such employment agreement and director agreement permit each of the respective parties thereto to terminate such agreements upon notice. If we lose this key employee and/or the services of our other director, our business will likely suffer and we may have to cease operations.
Recruiting and retaining qualified scientific, clinical, manufacturing and sales and marketing personnel will also be critical to our future success, as we continue to develop PRP and grow as a company. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors, including our scientific co-founders, may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.
| 19 |
We expect to expand our development, regulatory and future sales and marketing capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of drug development, regulatory affairs and sales and marketing. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The physical expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
We have identified material weaknesses in our internal control over financial reporting that, if not properly remediated, could result in material misstatements in our consolidated financial statements in future periods.
In connection with the audits of our financial statements for the fiscal years ended June 30, 2018 and 2017, we identified certain deficiencies relating to our internal control over financial reporting that constitute a material weakness under standards established by the Public Company Accounting Oversight Board (the “PCAOB”). The PCAOB defines a material weakness as a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. A deficiency in internal control exists when the design or operation of a control does not allow management or employees, in the normal course of performing their assigned functions, to prevent or detect misstatements on a timely basis.
The following material weaknesses in our internal control over financial reporting continued to exist at December 31, 2018:
| ● | we do not have written documentation of our internal control policies and procedures. Written documentation of key internal controls over financial reporting is a requirement of Section 404 of the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”); | |
| ● | we do not have sufficient segregation of duties within accounting functions, which is a basic internal control. Due to our limited size and early stage nature of operations, segregation of all conflicting duties may not always be possible and may not be economically feasible; however, to the extent possible, the initiation of transactions, the custody of assets and the recording of transactions should be performed by separate individuals; | |
| ● | lack of independent audit committee of our board of directors; and | |
| ● | insufficient monitoring and review controls over the financial reporting closing process, including the lack of individuals with current knowledge of U.S. GAAP. |
We outsource the functions that would normally be performed by a principal financial officer to assist us in implementing the necessary financial controls over the financial reporting and the utilization of internal management and staff to effectuate these controls.
We believe that these material weaknesses primarily relate, in part, to our lack of sufficient staff with appropriate training in U.S. GAAP and U.S. Securities and Exchange Commission (the “SEC”) rules and regulations with respect to financial reporting functions, and the lack of robust accounting systems, as well as the lack of sufficient resources to hire such staff and implement these accounting systems.
We
plan to take a number of actions in the future to correct these material weaknesses including, but not limited to, establishing
an audit committee of our board of directors comprised of at least two independent directors, adding experienced accounting and
financial personnel and retaining third-party consultants to review our internal controls and recommend improvements, subject
to receiving sufficient additional capital. If we receive sufficient capital, we hope to hire a part- or full-time chief financial
officer as the first step in building out our accounting department. We will need to take additional measures to fully mitigate
these issues, and the measures we have taken, and expect to take, to improve our internal controls may not be sufficient to (1)
address the issues identified, (2) ensure that our internal controls are effective or (3) ensure that the identified material
weakness or other material weaknesses will not result in a material misstatement of our annual or interim financial statements.
In addition, other material weaknesses may be identified in the future. If we are unable to correct deficiencies in internal controls
in a timely manner, our ability to record, process, summarize and report financial information accurately and within the time
periods specified in the rules and forms of the SEC will be adversely affected. This failure could negatively affect the market
price and trading liquidity of our common stock, cause investors to lose confidence in our reported financial information, subject
us to civil and criminal investigations and penalties, and generally materially and adversely impact our business and financial
condition.
| 20 |
We do not have any independent directors, which represents a potential conflict of interest, and helps create a material weakness in our disclosure controls and procedures as well as our internal control over financial reporting.
We do not have any independent directors, and no audit or compensation committees that in a larger company would be expected to be comprised of independent directors. The functions of these committees, as well as other important functions that would normally be carried out by independent directors, are performed by our directors, one of whom also serves as principal executive and financial officer of the Company, resulting in an inherent and obvious conflict of interest.
Also, our lack of independent directors and an audit committee necessitates that we do not currently have a director who qualifies as an audit committee financial expert. This fact, together with our additional lack of in-house accounting personnel knowledgeable in debt and equity transactions and our extremely small administrative staff that makes it impossible to segregate critical duties, combine to create material weaknesses in both our disclosure controls and procedures and our internal control over financial reporting.
If we fail to implement and maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud.
Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us conducted in connection with Section 404 of the Sarbanes-Oxley Act, or the subsequent testing by our independent registered public accounting firm, if and when required, may reveal additional deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our consolidated financial statements or identify other areas for further attention or improvement. If in the future we identify other material weaknesses in our internal control over financial reporting, including at some of our acquired companies, if we are unable to comply with the requirements of Section 404 in a timely manner or assert that our internal control over financial reporting is effective, or if our independent registered public accounting firm is unable to express an opinion as to the effectiveness of our internal control over financial reporting, investors may lose confidence in the accuracy and completeness of our financial reports and the market price of our common stock could be negatively affected, and we could become subject to investigations by the stock exchange on which our securities are then listed, the SEC, or other regulatory authorities, which could require additional financial and management resources. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our common stock.
Additionally, we currently do not have an internal audit group nor an audit committee of our board of directors, and we will eventually need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge to have effective internal controls for financial reporting.
We will continue to incur significant increased costs as a result of operating as a public company.
As a public company, we will continue to incur significant legal, accounting and other expenses. For example, we are subject to mandatory reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which require, among other things, that we continue to file with the SEC annual, quarterly and current reports with respect to our business and financial condition. We have incurred and will continue to incur costs associated with the preparation and filing of these SEC reports. In addition, the Sarbanes-Oxley Act, as well as rules subsequently implemented by the SEC, the Dodd-Frank Wall Street Reform and Consumer Protection Act (the “Dodd-Frank Act”) and national stock exchanges have imposed various other requirements on public companies. Stockholder activism, the current political environment and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact (in ways we cannot currently anticipate) the manner in which we operate our business. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations have and will continue to increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we will incur additional expense to increase our director and officer liability insurance.
In addition, if and when we cease to be a smaller reporting company and become subject to Section 404(b) of the Sarbanes-Oxley Act, we will be required to furnish an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with Section 404 within the prescribed time period, we will continue to be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to dedicate substantially greater internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that our independent registered public accounting firm, when required, will not be able to conclude within the prescribed timeframe that our internal control over financial reporting is effective as required by Section 404. This could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
| 21 |
Judgments that our stockholders obtain against us may not be enforceable.
Substantially all of our assets are located outside of the United States. In addition, our Chief Executive Officer, James Nathanielsz, resides in Australia and our other director, Dr. Julian Kenyon, resides in the UK. As a result, it may be difficult for you to effect service of process within the United States upon these persons. It is uncertain whether the courts of Australia or the UK would recognize or enforce judgments of the United States or state courts against us or such persons predicated upon the civil liability provisions of the laws of the United States or any state.
RISKS RELATED TO OUR COMMON STOCK
The market price of our common stock may continue to be highly volatile, you may not be able to resell your shares at or above the public offering price and you could lose all or part of your investment.
The trading price of our common stock may continue to be highly volatile. For example, the closing price of our stock during the period from August 24, 2018 to September 5, 2018, fluctuated between a low of $0.004 and a high of $0.2599. Our stock price could continue to be subject to wide fluctuations in response to a variety of factors, including the following:
| ● | actual or anticipated results of our clinical trials; | |
| ● | actions of securities analysts who initiate or maintain coverage of us, changes in financial estimates by any securities analysts who follow our company, or our failure to meet these estimates or the expectations of investors; | |
| ● | issuance of our equity and/or debt securities, or disclosure or announcements relating thereto; | |
| ● | additional shares of our common stock being sold into the market by us or our existing stockholders and/or holders of convertible debt or the anticipation of such sales; | |
| ● | stock market valuations of companies in our industry; | |
| ● | price and volume fluctuations in the overall stock market, including as a result of trends in the economy as a whole; | |
| ● | lawsuits threatened or filed against us; | |
| ● | regulatory developments in the United States and foreign countries applicable to biotech and biopharma companies; and | |
| ● | other events or factors, including those resulting from war or incidents of terrorism, or responses to these events. |
In addition, the stock market in general, and the OTCQB in particular, has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market, clinical trial results and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance.
Currently there is a limited public market for our common stock, and we cannot predict the future prices or the amount of liquidity of our common stock.
Currently, there is a limited public market for our common stock. Our common stock is traded on the OTCQB under the symbol “PPCB.” However, the OTCQB is not a liquid market in contrast to the major stock exchanges. We cannot assure you as to the liquidity or the future market prices of our common stock if a market does develop. If an active market for our common stock does not develop, the fair market value of our common stock could be materially adversely affected. We cannot predict the future prices of our common stock.
The designation of our common stock as a “penny stock” would limit the liquidity of our common stock.
Our common stock may be deemed a “penny stock” (as that term is defined under Rule 3a51-1 of the Exchange Act) in any market that may develop in the future. Generally, a “penny stock” is a common stock that is not listed on a securities exchange and trades for less than $5.00 a share. Prices often are not available to buyers and sellers and the market may be very limited. Broker-dealers who sell penny stocks must provide purchasers of these stocks with a standardized risk-disclosure document prepared by the SEC. The document provides information about penny stocks and the nature and level of risks involved in investing in the penny stock market. A broker must also provide purchasers with bid and offer quotations and information regarding broker and salesperson compensation and make a written determination that the penny stock is a suitable investment for the purchaser and obtain the purchaser’s written agreement to the purchase. Many brokers choose not to participate in penny stock transactions. Because of the penny stock rules, there may be less trading activity in any market that develops for our common stock in the future and stockholders are likely to have difficulty selling their shares.
| 22 |
Because our directors and officers currently and for the foreseeable future will continue to control our Company, it is not likely that you will be able to elect directors or have any say in the policies of our Company.
Our stockholders are not entitled to cumulative voting rights. Consequently, the election of directors and all other matters requiring stockholder approval will be decided by majority vote. Our directors and officers beneficially own less than 1.0% of our outstanding common stock. In addition, our chief executive officer and chief financial officer beneficially owns all of our preferred stock, which entitles him, as a holder of Series A preferred stock, to vote on all matters submitted or required to be submitted to a vote of the stockholders, except election and removal of directors, and each share entitles him to five hundred votes per share of Series A preferred stock, and as a holder of Series B preferred stock, to voting power equivalent of the number of votes equal to the total number of shares of common stock outstanding as of the record date for the determination of stockholders entitled to vote at each meeting of our stockholders and entitled to vote on all matters submitted or required to be submitted to a vote of our stockholders. Due to such a disproportionate voting power, new investors will not be able to affect a change in our business or management, and therefore, stockholders would have limited recourse as a result of decisions made by management.
Moreover, this preferred stock ownership may discourage a potential acquirer from making a tender offer or otherwise attempting to obtain control of us, which in turn could reduce our stock price or prevent our stockholders from realizing a premium over our stock price.
In the future, we may issue additional preferred stock without the approval of our stockholders, which could make it more difficult for a third party to acquire us and could depress our stock price.
Our board of directors may, and has in the past, issue, without a vote of our stockholders, one or more series of preferred stock with such rights and preferences as it determines. This could permit our board of directors to issue preferred stock to investors who support us and our management and permit our management to retain control of our business. Additionally, issuance of preferred stock could block an acquisition which could result in both a drop in our stock price and a decline in interest of our common stock.
Since we intend to retain any earnings for development of our business for the foreseeable future, you will likely not receive any dividends for the foreseeable future, and capital appreciation, if any, will be the source of gain for our stockholders.
We have never declared or paid any cash dividends or distributions on our capital stock. We currently intend to retain our future earnings to support operations and to finance expansion and therefore we do not anticipate paying any cash dividends on our common stock in the foreseeable future. As a result, capital appreciation, if any, of our common stock will be the sole source of gain for our stockholders for the foreseeable future.
Future sales and issuances of our capital stock or rights to purchase capital stock could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to decline.
We will likely issue additional securities in the future and such future sales and issuances of our capital stock or rights to purchase our capital stock could result in substantial dilution to our existing stockholders. We may sell common stock, convertible securities and other equity securities in one or more transactions at prices and in a manner as we may determine from time to time. If we sell any such securities in subsequent transactions, our stockholders may be materially diluted. New investors in such subsequent transactions could gain rights, preferences and privileges senior to those of holders of our common stock.
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
Section 382 (“Section 382”) of the Internal Revenue Code of 1986, as amended (the “Code”), contains rules that limit the ability of a company that undergoes an ownership change to utilize its net operating losses (“NOLs”) and tax credits existing as of the date of such ownership change. Under the rules, such an ownership change is generally any change in ownership of more than 50% of a company’s stock within a rolling three-year period. The rules generally operate by focusing on changes in ownership among stockholders considered by the rules as owning, directly or indirectly, 5% or more of the stock of a company and any change in ownership arising from new issuances of stock by the company. As a result of this Section 382 limitation, any ownership changes as defined by Section 382 may limit the amount of NOL carryforwards that could be utilized annually to offset future taxable income.
As a smaller reporting company, we are subject to scaled disclosure requirements that may make it more challenging for investors to analyze our results of operations and financial prospects.
As a “smaller reporting company,” we (i) are able to provide simplified executive compensation disclosures in our filings, (ii) are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal control over financial reporting and (iii) have certain other decreased disclosure obligations in our filings with the SEC, including being required to provide only two years of audited financial statements in annual reports. Consequently, it may be more challenging for investors to analyze our results of operations and financial prospects.
| 23 |
We will remain a smaller reporting company until the beginning of a fiscal year in which we had a public float of $250 million held by non-affiliates as of the last business day of the second quarter of the prior fiscal year, assuming our common stock is registered under Section 12 of the Exchange Act on the applicable evaluation date. Even if we remain a smaller reporting company, if our public float exceeds $75, we will become subject to the provisions of Section 404(b) of the Sarbanes-Oxley Act.
RISKS RELATING TO OUR EQUITY LINE WITH OASIS CAPITAL
Resales of shares purchased by Oasis Capital under the Equity Purchase Agreement may cause the market price of our common stock to decline.
Subject to the terms and conditions of the Equity Purchase Agreement with Oasis Capital (the “Equity Purchase Agreement”), we have the right to “put,” or sell, at our discretion, up to $10,000,000 worth of shares of our common stock to Oasis Capital. Unless terminated earlier, Oasis Capital’s purchase commitment will automatically terminate on the earlier of the date on which Oasis Capital shall have purchased shares pursuant to the Equity Purchase Agreement for an aggregate purchase price of $10,000,000 or February 25, 2022. This arrangement is also sometimes referred to herein as the “Equity Line” or the “Oasis Equity Line.” The shares to be issued to Oasis Capital pursuant to the Equity Purchase Agreement will be purchased at a price equal to 87.5% of the “Market Price,” which is defined as the one lowest daily volume weighted average traded price on the OTCQB, as reported by Bloomberg Finance L.P. or Quotestream, during the five trading days immediately following the date Oasis Capital receives the Put Shares via deposit/withdrawal at custodian share transfer method (“DWAC”) associated with the applicable put notice (the “Pricing Period”), which in most circumstances will be the trading day immediately following the date that a put notice is delivered to Oasis Capital (the “Put Date”). Oasis Capital may have the financial incentive to sell the shares of our common stock issuable under the Equity Purchase Agreement in advance of or upon receiving such shares and to realize the profit equal to the difference between the discounted price and the current market price of the shares. This may cause the market price of our common stock to decline.
The foregoing description of the terms of the Equity Purchase Agreement does not purport to be complete and is subject to and qualified in its entirety by reference to the Equity Purchase Agreement itself, which is filed as an exhibit to the registration statement, of which this prospectus forms a part of.
Puts under Equity Purchase Agreement may cause dilution to existing stockholders.
From time to time during the term of the Equity Purchase Agreement, and at our sole discretion, we may present Oasis Capital with a put notice requiring Oasis Capital to purchase shares of our common stock. We have the ability to sell up to $10 million in shares of our common stock (or approximately 666.67 million shares assuming a purchase price of $0.015 per share, the closing price of our shares of common stock on the OTCQB on February 14, 2019) under the Equity Purchase Agreement, subject to certain limits, and provided that, among other things, such shares are registered by us for resale by Oasis Capital. Pursuant to this registration statement of which this prospectus is a part, we are registering a total of 111,203,954 shares that Oasis Capital has agreed to purchase from us pursuant to the terms and conditions of an Equity Purchase Agreement. Such share amount represents one-third of the shares of our common stock held by non-affiliates of our Company as February 14, 2019. Our ability to sell any additional shares to Oasis Capital under the Equity Purchase Agreement will be contingent on our ability to prepare and file one or more additional registration statements registering the resale of such additional shares.
As a result, if we sell and issue any shares to Oasis Capital under the Equity Purchase Agreement, our existing stockholders will experience immediate dilution upon the purchase of any of the shares by Oasis Capital. Oasis Capital may resell some, if not all, of the shares that we issue to it under the Equity Purchase Agreement, and subject to certain volume limitations, the commitment fee shares, and such sales could cause the market price of our common stock to decline significantly. To the extent of any such decline, any subsequent puts would require us to issue and sell a greater number of shares to Oasis Capital in exchange for each dollar of the put amount. Under these circumstances, the existing stockholders of our company will experience greater dilution. The effect of this dilution may, in turn, cause the price of our common stock to decrease further, both because of the downward pressure on the stock price that would be caused by a large number of sales of our shares into the public market by Oasis Capital, and because our existing stockholders may disagree with a decision to sell shares to Oasis Capital at a time when our stock price is low, and may in response decide to sell additional shares, further decreasing our stock price. If we draw down amounts under the Equity Line when our share price is decreasing, we will need to issue more shares to raise the same amount of funding.
| 24 |
There is no guarantee that we will satisfy the conditions to the Equity Purchase Agreement.
Although the Equity Purchase Agreement provides that we can require Oasis Capital to purchase, at our discretion, up to $10,000,000 worth of shares of our common stock in the aggregate, our ability to put shares to Oasis Capital and obtain funds when requested is limited by the terms and conditions of the Equity Purchase Agreement, including restrictions on when we may exercise our put rights, restrictions on the amount we may put to Oasis Capital at any one time, which is determined in part by the trading volume of our common stock, and a limitation on our ability to put shares to Oasis Capital to the extent that it would cause Oasis Capital to beneficially own more than 9.99% of the outstanding shares of our common stock.
We may not have access to the full amount available under the Equity Purchase Agreement with Oasis Capital.
Our ability to draw down funds and sell shares under the Equity Purchase Agreement requires that a registration statement be declared effective and continue to be effective registering the resale of shares issuable under the Equity Purchase Agreement. The registration statement of which this prospectus is a part registers the resale of 111,203,954 shares of our common stock issuable under the Equity Line. Our ability to sell any additional shares under the Equity Purchase Agreement will be contingent on our ability to prepare and file one or more additional registration statements registering the resale of such additional shares. These registration statements (and any post-effective amendments thereto) may be subject to review and comment by the staff of the SEC, and will require the consent of our independent registered public accounting firm. Therefore, the timing of effectiveness of these registration statements (and any post-effective amendments thereto) cannot be assured. Even if we are successful in causing one or more registration statements registering the resale of some or all of the shares issuable under the Equity Purchase Agreement to be declared effective by the SEC in a timely manner, we may not be able to sell the shares unless certain other conditions are met. For example, we might have to increase the number of our authorized shares in order to issue the shares to Oasis Capital. Increasing the number of our authorized shares will require board and stockholder approval. Accordingly, because our ability to draw down any amounts under the Equity Purchase Agreement with Oasis Capital is subject to a number of conditions, there is no guarantee that we will be able to draw down all of the proceeds of $10,000,000 under the Equity Purchase Agreement.
***
The risks above do not necessarily comprise of all those associated with an investment in our Company. This registration statement of which this prospectus is a part contains forward looking statements that involve unknown risks, uncertainties and other factors that may cause our actual results, financial condition, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by such forward looking statements. Factors that might cause such a difference include, but are not limited to, those set out above.
CAUTIONARY STATEMENT ON FORWARD-LOOKING STATEMENTS
This prospectus may contain certain “forward-looking” statements as such term is defined by the SEC in its rules, regulations and releases, which represent the registrant’s expectations or beliefs, including but not limited to, statements concerning our operations, economic performance, financial condition, growth and acquisition strategies, investments, and future operational plans. For this purpose, any statements contained herein that are not statements of historical fact may be deemed to be forward-looking statements. Without limiting the generality of the foregoing, words such as “may,” “will,” “expect,” “believe,” “anticipate,” “intent,” “could,” “would,” “should,” “estimate,” “might,” “plan,” “predict” or “continue” or the negative or other variations thereof or comparable terminology are intended to identify forward-looking statements. These statements by their nature involve substantial risks and uncertainties, certain of which are beyond our control, and actual results may differ materially depending on a variety of important factors, including uncertainty related to the discovery, development and commercialization of our product candidates, protection of our intellectual property, governmental regulation, the operations of our Company and our subsidiaries, managing and maintaining growth, volatility of our stock price, and any other factors discussed in this and our other filings with the SEC.
These risks and uncertainties and other factors include, but are not limited to those set forth under the section captioned “Risk Factors” of this prospectus. Given these risks and uncertainties, readers are cautioned not to place undue reliance on our forward-looking statements. All subsequent written and oral forward-looking statements attributable to us or to persons acting on our behalf are expressly qualified in their entirety by these cautionary statements. Except as otherwise required by applicable law, we undertake no obligation to publicly update or revise any forward-looking statements or the risk factors described in this prospectus or in the documents we incorporate by reference, whether as a result of new information, future events, changed circumstances or any other reason after the date of this prospectus.
| 25 |
This prospectus contains forward-looking statements, including statements regarding, among other things:
| ● | our ability to continue as a going concern; | |
| ● | our anticipated needs for working capital; | |
| ● | our ability to successfully develop PRP, our lead product candidate; | |
| ● | our ability to reach research and development milestones as planned and within proposed budgets; | |
| ● | our current reliance on substantial debt financing; | |
| ● | our ability to repay current debt in cash and obtain adequate new financing; | |
| ● | our dependence on third parties for services; | |
| ● | our dependence on key executives; | |
| ● | our ability to control costs; | |
| ● | our ability to successfully implement our expansion strategies; | |
| ● | our ability to successfully develop and market our technologies; | |
| ● | our ability to obtain and maintain patent protection; | |
| ● | our ability to recruit employees with regulatory, accounting and finance expertise; | |
| ● | the impact of government regulations, including United States Food and Drug Administration (the “FDA”) regulations; | |
| ● | the impact of any future litigation; | |
| ● | the availability of capital; and | |
| ● | changes in economic, business and competitive conditions; |
Actual events or results may differ materially from those discussed in forward-looking statements as a result of various factors, including, without limitation, the risks, uncertainties and other factors outlined in the section captioned “Risk Factors” of this prospectus and matters described in this prospectus generally. In light of these risks and uncertainties, there can be no assurance that the forward-looking statements contained in this prospectus will in fact occur. We caution you not to place undue reliance on these forward-looking statements. In addition to the information expressly required to be included in this prospectus, we will provide such further material information, if any, as may be necessary to make the required statements, in light of the circumstances under which they are made, not misleading. All subsequent written and oral forward-looking statements attributable to our Company or to persons acting on our behalf are expressly qualified in their entirety by these cautionary statements. Except as required by law, we do not intend to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
We will not receive any proceeds from the sale of the shares of our common stock by the selling stockholder. However, we will receive proceeds from the sale of the shares of our common directly to Oasis Capital pursuant to the Equity Purchase Agreement. We will use these proceeds for general corporate and working capital purposes, or for other purposes that our board of directors, in its good faith, deems to be in the best interest of our Company. We have agreed to bear the expenses relating to the registration of the offer and resale by the selling stockholder of the shares being offered hereby.
DETERMINATION OF OFFERING PRICE
The selling stockholder will determine at what price it may sell the shares of our common stock offered by this prospectus, and such sales may be made at prevailing market prices, at prices related to the prevailing market price or at privately negotiated prices.
The selling stockholder may offer and resale of up to 111,203,954 shares of our common stock, par value $0.001 per share, pursuant to this prospectus. Such shares represent shares of our common stock that Oasis Capital has agreed to purchase from us pursuant to the terms and conditions of an Equity Purchase Agreement we entered into with Oasis Capital on February 25, 2019, which are described below.
| 26 |
Equity Purchase Agreement and Registration Rights Agreement with Oasis Capital, LLC
Subject to the terms and conditions of the Equity Purchase Agreement, we have the right to “put,” or sell, up to $10,000,000 worth of shares of our common stock to Oasis Capital. Unless terminated earlier, Oasis Capital’s purchase commitment will automatically terminate on the earlier of the date on which Oasis Capital shall have purchased shares pursuant to the Equity Purchase Agreement for an aggregate purchase price of $10,000,000 or February 25, 2022. We have no obligation to sell any shares under the Equity Purchase Agreement.
As provided in the Equity Purchase Agreement, we may require Oasis Capital to purchase shares of our common stock from time to time by delivering a put notice to Oasis Capital specifying the total number of shares to be purchased (such number of shares multiplied by the purchase price described below, the “Investment Amount”); provided there must be a minimum of ten trading days between delivery of each put notice. We may determine the Investment Amount, provided that such amount may not be more than 250% of the average daily trading volume in dollar amount for our common stock during the 10 trading days preceding the date on which we deliver the applicable put notice. Additionally, such amount may not be higher than $1,000,000. Oasis Capital will have no obligation to purchase shares under the Equity Line to the extent that such purchase would cause Oasis Capital to own more than 9.99% of our common stock.
For each share of the our common stock purchased under the Equity Line, Oasis Capital will pay a purchase price (the “Purchase Price”) equal to 87.5% of the “Market Price,” which is defined as the one lowest daily volume weighted average traded price on the OTCQB, as reported by Bloomberg Finance L.P. or Quotestream, during the five trading days immediately following the date Oasis Capital receives the Put Shares via DWAC associated with the applicable put notice, which in most circumstances will be the trading day immediately following the date that a put notice is delivered to Oasis Capital. On the settlement date, Oasis Capital will purchase the applicable number of shares of our common stock subject to customary closing conditions, including without limitation a requirement that a registration statement remain effective registering the resale by Oasis Capital of the shares to be issued under the Equity Line as contemplated by the Registration Rights Agreement described below. Oasis Capital may not assign its rights or obligations under the Equity Purchase Agreement and the Equity Purchase Agreement is not transferable and any benefits attached thereto may not be assigned.
In connection with the Equity Purchase Agreement, on February 25, 2019, we also entered into Registration Rights Agreement (the “Registration Rights Agreement,” and together with the Purchase Agreement, are referred to herein as the “Transaction Documents”) with Oasis Capital requiring us to prepare and file a registration statement registering the resale by Oasis Capital of the Put Shares (as defined below) by March 1, 2019, and to use commercially reasonable efforts to cause such registration statement to become effective, and to keep such registration statement effective until (i) the date when Oasis Capital may sell all of the shares that we may put to Oasis Capital (the “Put Shares”) under the Equity Purchase Agreement without any restrictions (including under Rule 144 without volume limitations), or (ii) the date when no Put Shares remain issuable under the Purchase Agreement. In accordance with the Registration Rights Agreement, on February 25, 2019, we filed the registration statement of which this prospectus is a part registering the resale by Oasis Capital of up to 111,203,954 Put Shares. This registration statement was declared effective by the SEC on ___________, 2019.
The 111,203,954 shares being offered pursuant to this prospectus by Oasis Capital represent approximately 33.33% of shares of our common stock issued and outstanding held by non-affiliates of our Company as of February 14, 2019 assuming the offering is fully subscribed.
Effective as of the Closing Date, we reserved approximately 666.67 million shares of our common stock from our authorized and unissued shares of common stock to provide for all issuances of shares of common stock under the Transaction Documents (in the event that we issue and sell the Put Shares up to $10,000,000, the maximum amount) and are required to reserve and keep available out of the authorized and unissued shares of our common stock a number of shares of our common stock at least three times the number of shares of common stock obtained by dividing the remaining balance on the maximum commitment amount by the Purchase Price. While we have the obligation to maintain such reserve while the Equity Purchase Agreement is effective, we do not have the obligation to sell any Put Shares to Oasis Capital. Oasis Capital also agreed that neither it nor any affiliate acting on Oasis Capital’s behalf or pursuant to any understanding with Oasis Capital, will execute any short sales during the three-year term of the Equity Purchase Agreement.
The Transaction Documents contain covenants, representations and warranties of our Company and Oasis Capital that are typical for transactions of this type. In addition, we and Oasis Capital have granted each other customary indemnification rights in connection with the Equity Purchase Agreement. The Equity Purchase Agreement may be terminated by us at any time.
We
intend to sell Oasis Capital periodically shares of our common stock under the Equity Purchase Agreement and Oasis Capital may,
in turn, sell such shares to investors in the market at the market price or at negotiated prices. This may cause our stock price
to decline, which will require us to issue increasing numbers of common shares to Oasis Capital to raise the intended amount of
funds, as our stock price declines.
| 27 |
Likelihood of Accessing the Full Amount of the Equity Line
Notwithstanding that the Equity Line is in an amount of $10,000,000, we anticipate that the actual likelihood that we will be able to access the full amount of the Equity Line is low due to several factors, including that our ability to access the Equity Line is impacted by our average daily trading volume, which may limit the maximum dollar amount of each put we deliver to Oasis Capital, and our stock price. Our use of the Equity Line will continue to be limited and restricted if our share trading volume or and market price of our stock continue at their current levels or decrease further in the future from the volume and stock prices reported over the past year. Further, if the price of our stock remains at $0.015 per share (which represents the last reported sale price of the shares of our common stock as reported on the OTCQB on February 15, 2019), the sale by Oasis Capital of all 111,203,954 of the Put Shares registered in this prospectus would mean we would receive only approximately $1,459,552 from our sale of the shares under the Equity Line. Our ability to issue shares in excess of the 111,203,954 shares covered by the registration statement of which this prospectus is a part will be subject to our filing a subsequent registration statement with the SEC and the SEC declaring it effective.
In addition, because our ability to deliver puts to Oasis Capital under the Equity Purchase Agreement is subject to a number of conditions, there is no guarantee that we will receive all or any portion of the $10,000,000 that is available to us under the Equity Line.
This prospectus covers the resale by the selling stockholder or its permitted transferees of up to 111,203,954 shares that may be issued by us to Oasis Capital under the Equity Purchase Agreement. Oasis Capital is an “underwriter” within the meaning of the Securities Act in connection with its resale of our common stock pursuant to this prospectus. The selling stockholder has not had any position or office, or other material relationship with us or any of our affiliates over the past three years. The following table sets forth certain information regarding the beneficial ownership of shares of common stock by the selling stockholder as of February 14, 2019 and the number of shares of our common stock being offered pursuant to this prospectus.
The table below (i) lists the selling stockholder and other information regarding the beneficial ownership (except with respect to the totals in Column 2, as determined under Section 13(d) of the Exchange Act and the rules and regulations thereunder) of our Common Stock by the selling stockholder; (ii) have been prepared based upon information furnished to us by the selling stockholder; and (iii) to our knowledge, is accurate as of the date of this prospectus. The selling stockholder may sell all, some or none of their shares in this offering. The selling stockholder identified in the table below may have sold, transferred or otherwise disposed of some or all of its shares since the date of this prospectus in transactions exempt from or not subject to the registration requirements of the Securities Act. Information concerning the selling stockholder may change from time to time and, if necessary, we will amend or supplement this prospectus accordingly and as required.
| Shares beneficially | Number
of shares to be beneficially owned and percentage of beneficial ownership after the offering(1)(2) | |||||||||||||||
| Name
of selling stockholder | owned as of the date of this prospectus(1) | Number of shares being offered | Number
of shares | Percentage
of class (3) | ||||||||||||
| Oasis Capital, LLC(4) | 0 | 111,203,954 | (5) | 0 | 0 | % | ||||||||||
| (1) | Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to shares of common stock. Shares of common stock subject to options and warrants currently exercisable, or exercisable within 60 days, are counted as outstanding for computing the percentage of the person holding such options or warrants but are not counted as outstanding for computing the percentage of any other person. | |
| (2) | The amount and percentage of shares of our common stock that will be beneficially owned by the selling stockholder after completion of the offering assume that they will sell all shares of our common stock being offered pursuant to this prospectus. | |
| (3) | Based on 333,896,577 shares of our common stock issued and outstanding as February 14, 2019. All shares of our common stock being offered pursuant to this prospectus by the selling stockholder are counted as outstanding for computing the percentage beneficial ownership of such selling stockholder. | |
| (4) | Adam Long has sole voting and investment control over shares owned by Oasis Capital. | |
| (5) | Represents one-third of the shares of our common stock held by non-affiliates of our Company as February 14, 2019. |
| 28 |
The selling stockholder or its permitted transferees may, from time to time, sell any or all of shares of our common stock covered hereby on the OTCQB, or any other stock exchange, market or trading facility on which the shares are traded or in private transactions. The selling stockholder may sell all or a portion of the shares being offered pursuant to this prospectus at fixed prices, at prevailing market prices at the time of sale, at varying prices or at negotiated prices. The selling stockholder may use any one or more of the following methods when selling securities:
| ● | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; | |
| ● | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; | |
| ● | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; | |
| ● | an exchange distribution in accordance with the rules of the applicable exchange; | |
| ● | privately negotiated transactions; | |
| ● | in transactions through broker-dealers that agree with the selling stockholder to sell a specified number of such securities at a stipulated price per security; | |
| ● | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; | |
| ● | a combination of any such methods of sale; or | |
| ● | any other method permitted pursuant to applicable law. |
The selling stockholder may also sell securities under Rule 144 under the Securities Act, if available, rather than under this prospectus.
Broker-dealers engaged by the selling stockholder may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the selling stockholder (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be negotiated, provided such amounts are in compliance with FINRA Rule 2121. Discounts, concessions, commissions and similar selling expenses, if any, that can be attributed to the sale of common stock will be paid by the selling stockholder and/or the purchasers.
Oasis Capital is an underwriter within the meaning of the Securities Act and any broker-dealers or agents that are involved in selling the shares may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Because Oasis Capital is an underwriter within the meaning of the Securities Act, it will be subject to the prospectus delivery requirements of the Securities Act.
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously engage in market making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution. In addition, the selling stockholder will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of securities of the common stock by the selling stockholder or any other person. We will make copies of this prospectus available to the selling security holders and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale.
Although Oasis Capital has agreed not to enter into any “short sales” of our common stock, sales after delivery of a put notice of a number of shares reasonably expected to be purchased under a put notice shall not be deemed a “short sale.” Accordingly, Oasis Capital may enter into arrangements it deems appropriate with respect to sales of shares of our common stock after it receives a put notice under the Equity Purchase Agreement so long as such sales or arrangements do not involve more than the number of put shares reasonably expected to be purchased by Oasis Capital under such put notice.
| 29 |
The following summary description of our capital stock is based on the provisions of our Certificate of Incorporation (as amended, the “Certificate of Incorporation”) and Bylaws and the applicable provisions of the General Corporation Law of the State of Delaware. This information is qualified entirely by reference to the applicable provisions of our Certificate of Incorporation, Bylaws and the General Corporation Law of the State of Delaware. Copies of our Certificate of Incorporation and Bylaws have been filed as exhibits to the registration statement of which this prospectus is a part. Also see “Where You Can Find More Information” section of this prospectus.
Authorized Capital Stock
Our authorized capital stock consists of 4,000,000,000 shares of common stock, $0.001 par value per share, and 1,500,005 shares of preferred stock, $0.01 par value per share. As of February 14, 2019, there were (i) 333,896,577 shares of our common stock issued and outstanding and (ii) 500,001 shares of our preferred stock issued and outstanding, consisting of 500,000 preferred shares designated as our Series A Preferred Stock and one preferred share designated as our Series B Preferred Stock.
As of February 14, 2019, we had 70 holders of record of our common stock, which excludes stockholders whose shares were held in nominee or street name by brokers. The actual number of common stockholders is greater than the number of record holders and includes stockholders who are beneficial owners, but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
Common Stock
Voting ― Holders of our common stock are entitled to one vote for each share held of record on all matters submitted to a vote of the stockholders, including the election of directors, and do not have cumulative voting rights. Accordingly, the holders of a majority of the shares of our common stock entitled to vote in any election of directors can elect all of the directors standing for election.
Dividends ― Subject to preferences that may be applicable to any then outstanding preferred stock, the holders of common stock are entitled to receive dividends, if any, as may be declared from time to time by our board of directors out of legally available funds.
Liquidation ― In the event of our liquidation, dissolution or winding up, holders of our common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities, subject to the satisfaction of any liquidation preference granted to the holders of any outstanding shares of preferred stock.
Rights and Preferences ― Holders of our common stock have no preemptive, conversion or subscription rights, and there are no redemption or sinking fund provisions applicable to our common stock. The rights, preferences and privileges of the holders of our common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of our preferred stock that we may designate and issue in the future.
Fully Paid and Nonassessable ― All of our outstanding shares of common stock are, and the shares of common stock to be issued in this offering will be, fully paid and nonassessable.
Preferred Stock
Our board of directors has the authority, without further action by the stockholders, to issue up to 1,500,005 shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, to fix the rights, preferences and privileges of the shares of each wholly unissued series and any qualifications, limitations or restrictions thereon and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding.
Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of the common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in our control that may otherwise benefit holders of our common stock and may adversely affect the market price of the common stock and the voting and other rights of the holders of common stock.
As of February 14, 2019, 500,001 shares of preferred stock were issued and outstanding, consisting of: (i) 500,000 preferred shares designated as Series A Preferred Stock (the “Series A Preferred Stock”) pursuant to our Certificate of Designation filed with the Secretary of State of the State of Delaware on December 9, 2014; and (ii) one preferred share designated as Series B Preferred Stock (the “Series B Preferred Stock”) pursuant to our Certificate of Designation filed with the Secretary of State of the State of Delaware on June 16, 2015. Up to five shares have been designated as Series B Preferred Stock.
| 30 |
Mr. Nathanielsz, our Chief Executive Officer, Chief Financial Officer, Acting Chairman, Secretary, Treasurer and a director, beneficially owns all of the shares of Series A Preferred Stock via North Horizon Pty Ltd., which entitles him, as a holder of Series A Preferred Stock, to vote on all matters submitted or required to be submitted to a vote of our stockholders, except election and removal of directors, and each share of Series A Preferred Stock entitles him to two votes per each outstanding share of Series A Preferred Stock. North Horizon Pty Ltd. is a Nathanielsz Family Trust. Mr. Nathanielsz has voting and investment power over these shares.
Mr. Nathanielsz directly beneficially owns such one share of Series B Preferred Stock. Each holder of outstanding shares of Series B Preferred Stock is entitled to voting power equivalent to the number of votes equal to the total number of shares of common stock outstanding as of the record date for the determination of stockholders entitled to vote at each meeting of stockholders of our Company and entitled to vote on all matters submitted or required to be submitted to a vote of the stockholders of the Company.
All of our issued and outstanding shares of preferred stock have no rights to dividends, profit sharing or liquidation preferences.
Authorized and Unissued Capital Stock
Delaware law does not require stockholder approval for any issuance of authorized shares. These additional shares may be used for a variety of corporate purposes, including future public offerings, to raise additional capital or to facilitate acquisitions.
One of the effects of the existence of unissued and unreserved common stock or preferred stock may be to enable our board of directors to issue shares to persons friendly to current management, which issuance could render more difficult or discourage an attempt to obtain control of our company by means of a merger, tender offer, proxy contest or otherwise, and thereby protect the continuity of our management and possibly deprive the stockholders of opportunities to sell their shares at prices higher than prevailing market prices.
Dividends
We have not paid any cash dividends to our shareholders. The declaration of any future cash dividends is at the discretion of our board of directors and depends upon our earnings, if any, our capital requirements and financial position, and general economic conditions. It is our present intention not to pay any cash dividends in the foreseeable future, but rather to reinvest earnings, if any, in our business operations.
Warrants
As of December 31, 2018, we had issued and outstanding warrants to purchase 29,517 shares of our common stock, with a weighted average exercise price per share of $9.53. See Note 6 – Stockholders’ Deficit to our unaudited condensed consolidated financial statements, included elsewhere in this prospectus, for a more detailed discussion of our issued and outstanding warrants.
Options
As of December 31, 2018, we had entered into agreements to grant options to purchase 572,000 shares of our common stock, with a weighted average exercise price per share of $7.50. See Note 6 – Stockholders’ Deficit to our unaudited condensed consolidated financial statements, included elsewhere in this prospectus, for a more detailed discussion of our issued and outstanding options.
Delaware Anti-Takeover Statute
We are subject to the provisions of Section 203 of the General Corporation Law of the State of Delaware regulating corporate takeovers. In general, Section 203 prohibits a publicly held Delaware corporation from engaging, under certain circumstances, in a business combination with an interested stockholder for a period of three years following the date the person became an interested stockholder unless:
| ● | prior to the date of the transaction, our board of directors approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; | |
| ● | upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, but not the outstanding voting stock owned by the interested stockholder, (1) shares owned by persons who are directors and also officers and (2) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| ● | at or subsequent to the date of the transaction, the business combination is approved by our board of directors and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock which is not owned by the interested stockholder. |
| 31 |
Generally, a business combination includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. An interested stockholder is a person who, together with affiliates and associates, owns or, within three years prior to the determination of interested stockholder status, did own 15% or more of a corporation’s outstanding voting stock. We expect the existence of this provision to have an anti-takeover effect with respect to transactions our board of directors does not approve in advance. We also anticipate that Section 203 may discourage attempts that might result in a premium over the market price for the shares of common stock held by stockholders.
The provisions of Delaware law and the provisions of our Certificate of Incorporation and Bylaws could have the effect of discouraging others from attempting hostile takeovers and, as a consequence, they might also inhibit temporary fluctuations in the market price of our common stock that often result from actual or rumored hostile takeover attempts. These provisions might also have the effect of preventing changes in our management. It is also possible that these provisions could make it more difficult to accomplish transactions that stockholders might otherwise deem to be in their best interests.
Bylaws
Provisions of our Bylaws may delay or discourage transactions involving an actual or potential change in our control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares or transactions that our stockholders might otherwise deem to be in their best interests. Therefore, these provisions could adversely affect the price of our common stock. Among other things, our Bylaws:
| ● | permit our board of directors to issue up to 1,500,005 shares of our preferred stock, with any rights, preferences and privileges as they may designate (including the right to approve an acquisition or other change in our control); | |
| ● | provide that the authorized number of directors may be changed only by resolution of the board of directors; | |
| ● | provide that all vacancies, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum; and | |
| ● | do not provide for cumulative voting rights (therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose). |
The amendment of any of these provisions, with the exception of the ability of our board of directors to issue shares of preferred stock and designate any rights, preferences and privileges thereto, would require approval by the holders of a majority of our then outstanding common stock.
OTC Markets - OTCQB Quotation
Our common stock is currently quoted on the OTC Markets - OTCQB market under the trading symbol “PPCB.”
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Action Stock Transfer Corporation. The transfer agent and registrar’s address is 2469 E. Fort Union Blvd., Suite 214, Salt Lake City, UT 84121.
Our consolidated financial statements as of and for the years ended June 30, 2018 and 2017, appearing in this prospectus and the registration statement of which it is a part, have been audited by Salberg & Company, P.A., an independent registered public accounting firm, as set forth in their report dated September 14, 2018 (which contains an explanatory paragraph regarding our ability to continue as a going concern) appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
| 32 |
Foley Shechter Ablovatskiy LLP has provided us with an opinion on the validity of the shares of our common stock being offered pursuant to this prospectus.
INTEREST OF NAMED EXPERTS AND COUNSEL
In November 2018, we issued 1,000,000 shares of our common stock to certain partners and associates of Foley Shechter Ablovatskiy LLP in consideration for general legal services provided by such firm to us. No expert named in the registration statement of which this prospectus forms a part as having prepared or certified any part thereof (or is named as having prepared or certified a report or valuation for use in connection with such registration statement) or counsel named in this prospectus as having given an opinion upon the validity of the securities being offered pursuant to this prospectus or upon other legal matters in connection with the registration or offering such securities was employed for such purpose on a contingency basis. Also, other than as set forth herein, at the time of such preparation, certification or opinion or at any time thereafter, through the date of effectiveness of such registration statement or that part of such registration statement to which such preparation, certification or opinion relates, no such person had, or is to receive, in connection with the offering, a substantial interest, direct or indirect, in our Company or any of our parents or subsidiaries. Nor was any such person connected with our Company or any of our parents or subsidiaries as a promoter, managing or principal underwriter, voting trustee, director, officer or employee.
| 33 |
Our Company
We are a development-stage healthcare company that is currently focused on developing new cancer treatments for patients suffering from pancreatic, ovarian and colorectal cancer. Utilizing our scientific and oncology consultants, we have developed a rational, composite formulation of anti-cancer compounds, which together exert a number of effects designed to control or prevent tumors from recurring and spreading through the body. Our lead product candidate, PRP, is a variation upon our novel formulation and involves pro-enzymes, the inactive precursors of enzymes. As a result of positive early indications of the anti-cancer effects of our technology, over the last year we have conducted successful pre-clinical studies on PRP and subject to us receiving adequate financing, hope to submit a clinical trial application in the 2019 calendar year. We intend to develop our PRP to treat early-stage cancer and pre-cancerous diseases and as a preventative measure for patients at risk of developing cancer based on genetic screening.
Key Research and Development Highlights:
| ● | Potential cancer treatment: We are developing PRP, an intravenous once-daily pro-enzyme treatment as a therapeutic option in cancer treatment and prevention. PRP is a combination of the pancreatic proenzymes trypsinogen and chymotrypsinogen. | |
| ● | Multiple mechanisms of action on cancerous or carcinogenic cells: PRP produces multiple effects on cancerous cells intended to inhibit tumor growth and potentially stop a tumor from spreading through the body. This is in contrast to current cancer treatments that lack sufficient efficacy to achieve a durable clinical response. As our research progresses, we intend to explore further these multiple mechanisms of action in order to identify opportunities to expand our intellectual property portfolio. Furthermore, we hope to uncover the molecular targets of the pro-enzymes to identify their potential for developing new compounds. | |
| ● | Encouraging data from patient treatment: We began our development efforts by analyzing scientific research undertaken over the last 15 years, including clinical data from patients in the UK and Australia. We concluded that there is at least indirect evidence that a formulation such as PRP may be an effective treatment against cancer and warranted further development. | |
| ● | Pre-Clinical Efficacy Studies: In November 2015, we completed animal efficacy studies in mice through our contract research partner, vivoPharm, demonstrating proof of concept in vivo. During the course of these studies, we discovered a new target therapeutic dose range using pro-enzymes for treating cancer. That month, we filed a patent application in support of this discovery, as described further herein. | |
| ● | Pre-Clinical Toxicology Studies: In October 2016, we completed an animal study for PRP, in which we evaluated its toxicokinetic parameters as well as its distribution and bioavailability, both before and after repeat dosages. We then initiated a second such study in December 2016. That study escalated the dosage levels in different phases and was completed in April 2017. We observed no major toxicological findings after PRP was administered by intravenous injection once daily throughout the study period. | |
| ● | Anticipated Clinical Trial Application: With the successful completion of the studies described above, we believe we have accumulated sufficient data to establish a safe and effective dosage level for PRP and advance our product development to the clinical stage. We are currently working with our manufacturer to create the finished product that will be part of our Investigational Medicinal Product Dossier to be submitted in connection with our anticipated first clinical trial for PRP, which we expect will be conducted in the UK, Europe or Australia. | |
| ● | Orphan Drug Designation: In June 2017, we received notification from the U.S. Food and Drug Administration (FDA) that PRP had been conferred Orphan Drug Designation. This special status is granted when a rare disease or condition is implicated and a potential treatment qualifies under the Orphan Drug Act and applicable FDA regulations. Orphan Drug status qualifies us for various development incentives, including protocol assistance, the potential for research grants, the waiver of future application fees, and tax credits for clinical testing if we choose to host future clinical trials in the U.S. | |
| ● | Unique intellectual property: In addition to our pre-clinical studies, we have also focused on building a significant portfolio of intellectual property around the use of pro-enzymes in the treatment of cancer, identifying new formulations, alternative routes of administration and potential new therapeutic targets. As described in greater detail within this S-1, we have filed numerous patent applications relating to PRP, several of which have been granted while others remain pending. In the U.S., we have been issued one patent to date (No. 9,636,359). Our most recent patent grant was granted in South Korea in January 2019. Our patent protection extends to both PRP’s mechanism of action and the new compositions of pro-enzymes. | |
| ● | Research and development expenses: During the six months ended December 31, 2018 and 2017, we have spent $150,970 and $1,598,468, respectively, and during the last two fiscal years ended June 30, 2018 and 2017, we have spent $1,825,728 and $971,769, respectively, on research and development expenses. Historically, we have assumed all of the costs associated with research and development. In September 2018, we entered into a two-year joint collaboration agreement with the Jaėn University, which is based in Andalucía, Spain, to assist us in expanding our product pipeline by discovering new compounds based on trypsinogen and chymotrypsinogen. |
| 34 |
Company History
We were originally incorporated in Melbourne, Victoria Australia on October 15, 2007 as Propanc PTY LTD and continue to be based in Camberwell, Victoria Australia. Since our inception, substantially all of our operations have been focused on the development of new cancer treatments targeting high-risk patients, particularly cancer survivors, who need a follow-up, non-toxic, long-term therapy designed to prevent the cancer from returning and spreading. We anticipate establishing global markets for our products.
On November 23, 2010, our Company was incorporated in the state of Delaware as Propanc Health Group Corporation. In January 2011, to reorganize our Company, we acquired all of the outstanding shares of Propanc PTY LTD on a one-for-one basis and Propanc PPY LTD became our wholly-owned subsidiary. Effective April 20, 2017, we changed our name to “Propanc Biopharma, Inc.” to better reflect our stage of operations and development. On the same date, we also effected a 1-for-250 reverse stock split whereby we (i) decreased the number of authorized shares of our common stock to 100,000,000 (ii) decreased the number of authorized shares of our preferred stock to 1,500,005 and (iii) decreased, by a ratio of 1-for-250 the number of retroactively issued and outstanding shares of our common stock.
On January 23, 2018, we filed a Certificate of Amendment to our Certificate of Incorporation to increase the number of authorized shares of our common stock from 100,000,000 to 400,000,000. On September 21, 2018, we filed a Certificate of Amendment to our Certificate of Incorporation to increase the number of authorized shares of our common stock from 400,000,000 to 4,000,000,000.
Important Milestones for Propanc
| ● | From the late 1990s, work from other scientists and clinicians, including Dr. Josef Novak in the U.S., and a since retired oncologist from the Czech Republic, Dr. Frantisek Trnka, shed new light on the therapeutic potential of Professor John Beard’s insights. Extensive laboratory work undertaken over a number of years by Novak and Trnka was reported in the journal Anticancer Research in 2005 in the paper entitled Pro-enzyme Therapy of Cancer. The conclusion of Novak and Trnka from this work was the discovery “that pro-enzyme therapy mandated first by John Beard nearly one hundred years ago, shows remarkable selective effects that result in growth inhibition of tumor cells with metastatic potential.” Today, these important scientific observations support our view that pro-enzymes are selective and effective in targeting malignant tumor cells and could become an effective tool in the fight against metastatic cancer. | |
| ● | In 2007, Dr. Julian Kenyon, Medical Director of the Dove Clinic in the UK, and Dr. Douglas Mitchell further developed the therapeutic concepts of Beard and identified strategies that could improve upon the therapeutic potential of Beard’s original ground-breaking work. A suppository formulation was developed by Mandeville Medicines in Buckinghamshire, UK, at the request of, and in consultation with, Drs. Kenyon and Mitchell, in an effort to improve on results reported in the literature pertaining to the potential therapeutic use of pro-enzymes in cancer treatment. Patients were first treated with the suppository formulation in April 2007 at The Dove Clinic in the UK, and in July 2007 at the Opal Clinic in Australia. Drs. Kenyon and Mitchell, through The Dove Clinic and Opal Clinic respectively, treated cancer patients in the United Kingdom and Australia with a suppository formulation of pro-enzymes. The treatment was undertaken under special UK and Australian regulatory provisions. In the UK it was undertaken under the regulations of the Medicines and Healthcare Products Regulatory Agency (the “MHRA”), designed for patients who have special clinical needs that cannot be met by licensed medicinal products, and in Australia under the Therapeutic Goods Administration (“TGA”) Special Access Scheme, a mechanism that provides for the import and/or supply of an unapproved therapeutic good for a single patient, on a case by case basis. In both jurisdictions, patients are permitted to receive treatment on an individual basis for compassionate use as long it is supplied by a recognized, licensed manufacturer who is able to meet certain guidelines for unapproved products, and individual case files are maintained for patients should the regulatory authorities require this information. No prior approval was required by either the MHRA or TGA prior to the commencement of treatment. No suppository formulation of the pro-enzymes was available and it was necessary for a novel suppository formulation to be manufactured specifically for these patients by a suitably licensed manufacturer. | |
| ● | Forty-six late stage cancer patients suffering from a range of malignancies in the UK and Australia received treatment with the pro-enzyme suppositories over periods of time ranging from one month to in excess of 17 months. Inspired by their observations in clinical practice, Drs. Kenyon and Mitchell resolved to develop pro-enzyme therapy for cancer patients worldwide. |
| 35 |
| ● | In late 2007, Drs. Kenyon and Mitchell and Mr. James Nathanielsz, our Chief Executive Officer and Chief Financial Officer, developed a strategy to commercialize the newly developed pro-enzyme formulation, now designated PRP. Propanc PTY LTD. was established in Australia as a vehicle to refine, develop and commercialize novel, patented pro-enzyme therapeutics for the treatment of cancer. | |
| ● | In 2008, our Scientific Advisory Board (the “Scientific Advisory Board”) comprising Professor John Smyth (Edinburgh University), Professor Klaus Kutz (Bonn University) and Professor Karrar Khan (De Montfort University) was established. Today, the expertise of the Scientific Advisory Board in oncology research and development will be relied upon as we initiate patient trials and advance our products down the requisite regulatory pathways to commercialize our pro-enzyme therapies. | |
| ● | In 2009, a retrospective review of the patient notes from the 46 patients treated in the UK and Australia with the pro-enzymes suppositories (as described above) was undertaken by Dr. Kenyon. This report was subject to analysis by Professor Klaus Kutz who, at the time of the review, was an independent consultant in clinical pharmacology and safety, specializing in oncology. Professor Kutz observed that no patients were reported as living for a period less than that predicted by the treating clinician and a number of terminally ill patients lived marginally longer than predicted, particularly those suffering from pancreatic, colorectal, ovarian and gastro-intestinal cancers. As a result of the observations made by Dr. Kenyon and Professor Kutz, we are targeting the development of pro-enzyme therapy for the treatment of colorectal and pancreatic cancers for clinical trials, and in the future targeting other cancer types as our product candidate progresses to commercialization. | |
| ● | In early 2008, a research collaborative partnership was established with Professor David Tosh at the Center for Regenerative Medicine, Department of Biology and Biochemistry at Bath University, to investigate the molecular mechanisms by which the pro-enzyme formulation is acting, which resulted in us filing two provisional patents a year later. We undertook additional scientific research with Professor Tosh, Dr. Macarena Perán, Department of Health Sciences at Jaén University, and Dr. Juan Antonio Marchal, Biopathology and Regenerative Medicine Institute at Granada University. Important anti-cancer effects of the pro-enzymes were discovered, including triggering cell necrosis (cell death) and apoptosis (programmed cell death) and significantly, the induction of cell differentiation (i.e. inducing cancer cells to exhibit normal cell behavior). This led to us increasing our intellectual property base and patent new pharmaceutical compositions designed to enhance the effects of pro-enzymes. Subsequently, two provisional patents were combined into one Patent Cooperation Treaty (“PCT”) Application, filed on October 22, 2010 (PCT Application), and then a year later, we completed a 30 month national phase filing deadline for an international patent and commenced entering the national phase in countries around the world. Thus far, we have received grant status in Australia, China, Japan, Indonesia, Israel, New Zealand, Singapore and South Africa and our application remains under examination in Brazil, Canada, the European Union, Malaysia, Mexico and the Republic of Korea. In the United States, one patent has been issued to date by the United States Patent and Trademark Office (No. 9,636,359) while another remains pending. We also have a second PCT application for our proenzyme composition that is pending as well two other applications filed and pending in Spain. | |
| ● | In late 2010, we made important discoveries and scientific observations, resulting in additional composition claims, which were included in the original PCT Application, further protecting the company’s pro-enzyme formulation. Collaboration with vivoPharm Pty Ltd. (“vivoPharm”), located in Melbourne, Australia, with research facilities in Hershey, Pennsylvania, United States, identified a highly synergistic ratio of the pro-enzymes when combined together, resulting in increased anti-cancer effects in several tumor cell lines. Furthermore, although α-Amylase was previously included in the early days of enzyme therapy and in the suppository formulation developed by Dr. Kenyon and Dr. Mitchell, after evaluating the synergistic interaction between the two pro-enzymes and α-Amylase, we concluded that α-Amylase did not contribute to the anti-tumor activity of the formulation, and so it was removed. By 2011, further work completed by vivoPharm confirmed the anti-metastatic effects of the newly combined ratio of the pro-enzymes in various cell line assays, and anti-angiogenic (inhibition of blood vessel formation) properties of the pro-enzyme treatment in mice. | |
| ● | At this time, we decided to access the U.S. markets in order to raise the capital needed to finance the Company’s pro-enzyme treatment for future preclinical testing and clinical trials. We incorporated as Propanc Health Group Corporation in the state of Delaware in November 2010 and in January 2011, we acquired all of the outstanding shares of Propanc PTY LTD on a one-for-one basis making and Propanc PTY LTD became our wholly-owned subsidiary. In mid-2012, our common stock began trading on the Over-the-Counter Bulletin Board and it currently trades on OTCQB. |
| 36 |
| ● | In May 2013, it was observed that pro-enzymes enforce the re-entry of cancer cells back into normal cellular pathways and this may represent a novel approach to the treatment of cancer. These findings were published in Cellular Oncology, a peer reviewed journal of the International Society for Cellular Oncology. | |
| ● | In 2014, after conducting a detailed strategic review of our scientific and preclinical research, our development team determined that parenteral drug administration is the preferred route for the Company’s lead product, PRP. This approach is expected to maximize results in future patient trials, by ensuring maximum exposure of the drug to the tumor site. | |
| ● | In mid-2015, Dr. Joseph Chalil joined our Scientific Advisory Board as an independent expert to provide advice on the Company’s drug development programs, in particular, our lead product, PRP. Dr. Chalil is a physician and executive at Boehringer Ingelheim, one of the world’s largest privately held pharmaceutical companies. | |
| ● | Between July 2015 and February 2016, several scientific research findings were announced demonstrating significant anti-tumor efficacy in several animal models, including pancreatic and ovarian cancers at higher doses when administering pro-enzymes by intravenous injection, dramatic suppression of cancer stems cells in cell culture by altering several key pathways involved with invasion and metastasis, and identification of a synergistic response in a broad range of cancer types including kidney, melanoma, brain, prostate, liver, uterine and lung cancers. | |
| ● | In 2016, we added additional members from our partner universities and hospital to our Scientific Advisory Board, including Dr. Macarena Perán, who is currently Reader in Anatomy at the University of Jaén in Spain, Professor Juan Antonio Marchal Corrales, Professor of Anatomy and Embryology at the Faculty of Medicine at the University of Granada, and Dr. Maria García, Head of Translational Research at the University Hospital of Granada. | |
| ● | In August 2016, we entered into a Manufacturing Services Agreement and Quality Assurance Agreement with Amatsigroup NV, formally known as Q-Biologicals NV, a contract manufacturing organization located in Belgium. Pursuant to the Manufacturing Services Agreement, Amatsigroup produces for us certain drug substances and product containing certain enzymes at its facility in Belgium. We use these substances and products for development purposes, including but not limited to future clinical trials. |
| ● | In October 2016, we completed an animal study for PRP, in which we evaluated its toxicokinetic parameters as well as its distribution and bioavailability, both before and after repeat dosages. We then initiated a second such study in December 2016. That study escalated the dosage levels in different phases and was completed in April 2017. We observed no major toxicological findings after PRP was administered by intravenous injection once daily throughout the study period. | |
| ● | On April 20, 2017, we changed our corporate name to “Propanc Biopharma, Inc.” to better reflect our stage of operations and development. | |
| ● | In June 2017, we received notification from the FDA that PRP had been granted Orphan Drug Designation, a special status that will enable us to qualify for tax credits for our future clinical trials, among other benefits. | |
| ● | In October 2017, we published key findings relating to a combination of two proenzymes trypsinogen and chymotrypsinogen A with potent in vitro and in vivo anti-tumor efficacy in Scientific Reports, a peer reviewed scientific journal covering all areas of the natural sciences. It was concluded that PRP could have relevant oncological clinical applications for the treatment of advanced or metastatic adenocarcinoma and advanced epithelial ovarian cancer. |
| ● | In February 2018, we announced allowance of our key patent application from the European Patent Office (EPO) covering a pharmaceutical composition for treating cancer comprising trypsinogen and chymotrypsinogen within the European Union. The allowed patent application is the first approval for the Company in the EU, which protects the Company’s lead product candidate, PRP, a solution for once-daily intravenous administration of a combination of two pancreatic proenzymes trypsinogen and chymotrypsinogen.
| |
| ● | In March 2018, we completed the successful reproduction run of the manufacturing process for the Company’s two drug substances trypsinogen and chymotrypsinogen. The successful reproduction run demonstrated scalability of our proprietary manufacturing process to enable routine production of the two active substances for PRP. The process was developed in collaboration with a European Contract Manufacturing Organization (CMO) experienced in the production of biopharmaceuticals.
| |
| ● | In July 2018, we entered national phase for two of our key patent applications from our intellectual property portfolio. The first patent application, which entered national phase in July 2018, describes a method to eradicate cancer stem cells, and a second patent application, covering proenzyme compositions for the treatment of solid tumors, recently completed national phase entry mid-July 2018. National phase is a process whereby applicants file a patent application in each individual jurisdiction or country, according to where intellectual property protection is sought. |
| 37 |
| ● | In September 2018, we presented at the 25th Annual NewsMakers in the Biotech Industry Conference held at the Millennium Broadway Hotel and Conference Center in New York, NY. This prestigious conference is sponsored by BioCentury, where only 45 companies are handpicked to present their stories to institutional investors in the biotech sector. At the conference, we discussed, among other things, recent scientific advancements of PRP and our ability to suppress the cancer stem cell population, which we plan to submit for publication to a peer reviewed scientific journal, and explained the current anticipated timelines for commencing our engineering run and full scale GMP manufacturing batch of PRP, emphasizing our management’s focus was to identify a suitable source of capital as we prepare for filling our drug product for clinical trials, as well as the goal of reducing our debt on the balance sheet by increasing equity investment.
| |
| ● | In September 2018, we entered into a two-year collaboration agreement with the University of Jaen to provide certain research services to us. In consideration of such services, we agreed to pay the university approximately 52,000 Euros ($60,762 USD) in year one and a maximum of 40,000 Euros ($46,740 USD) in year two. Additionally, in exchange for full ownership of the intellectual property we agreed to pay royalties of 2% of net revenues to the University. | |
| ● | In December 2018, we appointed LaVoieHealthScience (“LHS”) as our communications agency of record. LHS is an integrated investor and public relations agency focused on advancing health and science innovations. We are partnering with LHS to develop corporate awareness of our Company as a health science innovator as we plan to transition our lead technology into human clinical trials in 2019. | |
| ● | In December 2018, we announced that our foundation patent application has been granted by the Office of the Controller General of Patents, Design and Trademarks, India. The foundation patent, which covers our lead product candidate, PRP, pioneers the discovery of a pharmaceutical composition for treating cancer via a combination of trypsinogen and/or chymotrypsinogen pancreatic proenzymes. As of December 31, 2018, the foundation patent has been granted in the USA, Europe (including Belgium, Czech Republic, Denmark, France, Germany, Ireland, Italy, the Netherlands, Portugal, Spain, Sweden, Switzerland/Liechtenstein, Turkey and the United Kingdom), China, Japan, Indonesia, Malaysia, Israel, Australia, New Zealand, Singapore, South Africa, Mexico, Republic of Korea, Hong Kong and more recently, India. It is presently under examination in Canada and Brazil. | |
| ● | In January 2019, we announced that a cooperation agreement has been entered into between the University of Jaén and our Company to commence the POP1 joint drug discovery program to be co-funded by both parties. The agreement coincides with the appointment of research scientist, Mr. Aitor González, to lead the drug discovery and research activities over the next 3 to 4 years. The objective of the program is to identify and develop suitable backup compounds to our lead product candidate, PRP.As part of the agreement, Macarena Perán, Ph.D. and Julian Kenyon, M.D. have been appointed as joint supervisors, representing the University and our Company, respectively. The program involves advancing new compounds through a drug screening process, followed by preclinical and early stage clinical development. As the drug candidate progresses along the development pathway, the collaboration will also involve the Universities of Granada and Jaén, as well as Granada and Almeria Hospitals, which are members of FIBAO, a Public Health Foundation, based in Granada, Spain, committed to assisting commercial partners with the development and commercialization of innovative technologies designed to benefit humankind. | |
| ● | In February 2019, we presented at the 2019 BIO CEO & Investor Conference held at the New York Marriott Marquis. This conference is one of the largest investor conferences focused on established and emerging publicly traded and select private biotech companies. As part of our presentation and one-on-one meetings, we provided a Company update for 2019, including our focus on the advancement of our lead product, PRP, in 2019. | |
| ● | Today, after deepening our scientific knowledge of the anti-cancer effects of pro-enzymes through our ongoing efforts with our research partners and strengthening our intellectual property portfolio by filing our patents in countries around the world, we believe we are ready to undertake human clinical trials and subject to receiving adequate financing, we hope to submit a clinical trial application in the 2019 calendar year. |
The Problem
In the early phases of tumor progression, cancer cells multiply near the site where their predecessors first began uncontrolled proliferation. The result, usually over a long period of time, is a primary tumor mass. Tumors often need to reach a large size before they make themselves apparent to the individual concerned, or the clinician screening for them.
Eventually,
tumors of substantial size may begin to compromise the functioning of organs in which they have arisen and begin to evoke symptoms.
In many cases, the effects on normal tissue function come from the physical pressure exerted by the expanding tumor masses. For
example, large tumors in the colon may obstruct digestion products through the lumen, or in the lungs, airways may be compromised.
| 38 |
As dangerous and threatening as these primary tumors are, they are ultimately responsible for only about 10% of deaths. A far greater threat often arises for the patient, even after a primary tumor has been identified and removed. This threat involves cancerous growths that are discovered at sites far removed from the locations in their bodies where their primary tumors first appeared. These cancerous growths, called metastases, are responsible for approximately 90% of patient deaths from cancer. Metastases are formed by cancer cells that have left the primary tumor mass and traveled by the body’s blood and lymphatic vessels (a vein-like vessel carrying lymph, or white blood cells, from the tissues) to seek new sites and form new colonies. For example, breast cancers often spawn metastatic colonies in many tissues throughout the body including the brain, liver, bones, and lungs.
For primary tumors that have not yet metastasized, current treatments for cancer can be effective in initially reducing tumor burden. However, for many forms of cancer, current treatments lack sufficient efficacy to achieve a long lasting clinical response. Therefore, a vast majority of patients who succumb to cancer are killed by tumors that have metastasized. According to the National Cancer Institute’s SEER Cancer Statistics Review (2001 – 2007), of the patients diagnosed with late stage metastatic breast cancer, only 23% are expected to live longer than five years. This is compared to a 98% five-year survival rate for an early stage breast cancer patient when the cancer is confined to the primary site.
The invasion-metastasis cascade
The great majority of life threatening cancers occur in epithelial tissues, yielding carcinomas. Epithelial cells generally have a multi-sided, uniform shape. They have well defined contact points with neighboring cells and a strong attachment to the underlying connective tissue, or stroma, which creates a framework for solid tumors in the body. Separating the two is the specialized type of extracellular matrix, known as the basement membrane.
By definition, carcinomas that originate on the epithelial side of the basement membrane are considered to be benign; as long as the cells forming them remain on the same side. However, many carcinomas acquire the ability to penetrate the basement membrane, and individual cancer cells or groups of cancer cells begin to invade the stroma. This mass of cells is now reclassified as malignant. Often, many pathologists and surgeons reserve the label “cancer” for those epithelial tumors that have acquired this invasive ability.
Thereafter, carcinoma cells may invade into lymphatic or blood microvessels. The latter may then transport these cancer cells to distant sites in the body where they may be trapped and subsequently form new metastases.
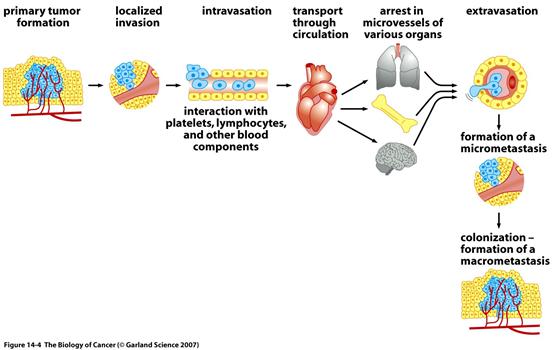
It
is important to note, that even before cells penetrate the basement membrane, they often stimulate angiogenesis (blood
vessel formation) on the stromal side of the membrane, by expressing angiogenic proteins through the porous barrier. Not only
does this enhance the ability of malignant cells to circulate into the blood, but also provides an important feedback loop for
the cancer cell to maintain its invasiveness.
| 39 |
Understanding the mechanism by which benign cells change to a malignant state is therefore pivotal to developing anti-cancer treatments that have sufficient efficacy to achieve a long lasting clinical response.
The epithelial-mesenchymal transition and associated loss of E-cadherin expression enable carcinoma cells to become invasive.
Epithelial cells can undergo a transformation to a different cell type, called mesenchymal cells, through a process called the epithelial-to-mesenchymal transition (“EMT”). Mesenchymal cells have an elongated spindle shape, lack orderly contacts with neighboring cells and can survive without contact with a surface or connective tissue. The EMT process is a series of events that normally occur during the development of tissues and organs prior to birth, and also apply to normal wound healing processes. However, the same EMT process can also be applied to epithelial cancer cells, or carcinomas. When epithelial carcinoma cells residing in a solid tumor undergo the EMT process, the resulting mesenchymal cancer cells can invade through local barriers and metastasize to other parts of the body.
In addition to becoming invasive and motile after undergoing the EMT process, the resulting mesenchymal cells have significantly increased resistance to current cancer treatments. For example, in Cancer Research in 2005, it was reported that lung cancer cells expressing mesenchymal biomarkers appeared to be resistant to Tarceva and other targeted anti-cancer agents when transplanted into mice.
At the center of this critical process for transforming benign cells into carcinomas, is the protein Epithelial Cadherin (“E-Cadherin”). In normal cells, E-cadherin is located in the membrane and involved in maintaining cell to cell contact, which is critical to normal function and structure of epithelial tissues. The individual E-Cadherin molecules are attached to the actin (scaffolding, or cytoskeleton structure) within the cell, anchored by β-catenin, a protein which helps form the junction between epithelial cells. As well as forming an anchor between epithelial cells, β-catenin is also involved in gene transcription, a process by which DNA (deoxyribose nucleic acid) is converted into RNA (ribose nucleic acid) within the nucleus of a cell for the purpose of producing new proteins normally associated with routine cell function.
In the case of tumors, when cells become invasive, E-Cadherin expression decreases substantially, and β-catenin becomes free within the cell, which may then migrate to the nucleus and induce expression of the EMT program. Furthermore, once cells undergo an EMT, they begin to produce their own cytokines (cell signaling molecules), such as Transforming Growth Factor β, (“TGF-β”). This protein plays a critical multi-functional role in promoting angiogenesis, immunosuppression (suppressing the immune system from recognizing and attacking cancer cells), and maintaining their mesenchymal cell structure for prolonged periods via a feedback mechanism. Studies also suggest that TGF-β works with β-catenin to cause epithelial cancer cells to undergo an EMT.
| 40 |
A study in the British Journal of Cancer in 2011 demonstrated that in cholangiocarcinoma (bile duct cancer) cell lines, treatment of TGF-β increased cell migration, invasion and mesenchymal changes. Furthermore, expression of E-cadherin and N-cadherin was measured from resected (cut out) specimens from extra-hepatic (outside the liver) cholangiocarcinoma patients. Patients with low E-cadherin expression had a significantly lower survival rate than patients with high E-cadherin expression. They concluded the cadherin switch via TGF-β induced EMT in extra-hepatic cholangiocarcinoma leads to cancer progression.
Conversely, in studies of several types of carcinoma cells that had lost E-cadherin expression, re-expression of this protein strongly suppressed the invasiveness and motility of these cancer cells.
Together, these observations indicate that E-Cadherin levels is a key determinant of the biological behavior of epithelial cancer cells and that the cell to cell contact constructed by E-cadherin molecules impede invasiveness and hence metastasis.
Our Solution
Our solution is to develop and commercialize a long-term therapy to prevent tumor recurrence and metastases, the main cause of patient death from cancer. We believe this problem can be addressed by developing a pro-enzyme formulation specifically targeting malignant carcinoma cells to create a long lasting clinical benefit to the patient.
Propanc’s Theory Pro-enzymes Regulate Cell Proliferation
More than 100 years ago, Professor John Beard, a comparative embryologist, made an observation that the pancreas develops in most vertebrates at the time when the placenta begins to slow its rate of growth. He hypothesized that enzymes produced by the developing pancreatic gland curtail trophoblastic invasion (a rare condition in which abnormal cells grow inside the uterus from tissue that forms after conception) and suggested that pancreatic extracts should have a similar inhibitory effect on invasive tumors.
Subsequently in the late 1990s, after following Professor Beard’s recommendations, Drs. Novak and Trnka hypothesized that administration of pro-enzymes, rather than the enzymes, was of crucial importance to the clinical effectiveness of the treatment approach first developed by Professor Beard, and that the precursor nature of the active enzymes may offer protection against numerous serpins (proteins which can inhibit pro-enzymes) in the blood.
As knowledge of tumor cell and molecular cell biology has increased over the years, our scientists and research partners have made important scientific discoveries identifying that pro-enzymes suppress the EMT program and induce cell differentiation, i.e., return cancerous cells towards normal cell behavior, or a benign state.
After more than 100 years, the initial observations made by Professor Beard may have a potential common link between embryogenesis and cancer, by which cells are able to become motile and invasive, via the EMT program, where the administration of pro-enzymes may regulate cell proliferation as a means to controlling carcinomas.
| 41 |
PRP
Our lead product, PRP, is a novel, patented formulation consisting of two pro-enzymes, trypsinogen and chymotrypsinogen, combined at a ratio of one-to-six (1:6), to be administered intravenously. After establishing proof of concept in vivo as described earlier, supplemented by laboratory research at the Universities of Jaén and Granada on the mechanism of action of the pro-enzyme mixture, evidence suggests PRP may be effective against a range of solid tumors.
Selectivity
Research published by Novak and Trnka in Anticancer Research (2005) suggests that the pro-enzymes in our product, trypsinogen and chymotrypsinogen, exhibit specificity for tumor cells and not normal cells. Once activated, they in turn activate Protease Activated Receptors Type 2 (“PAR2”), which are located on the cell membrane and involved with cancer cell proliferation. Activation of PAR2 results in a cascade of intracellular activities, including activation of a major component of the cell which controls its structure and architecture, the actin cytoskeleton. In a cancer cell, pro-enzymes have the effect of converting globular actin into filamentous actin, which causes the cell structure to collapse and induce cell death. This reduces tumor volume and is often seen in clinical practice.
Anti-Cancer Effects and Mechanism of Action
PRP consists of pro-enzymes which are known to influence a number of pathways critical for cancer cells to invade, grow and metastasize. Research published in collaboration with our research partners at Jaėn and Granada Universities in the Journal of Cellular Oncology in 2013 shows the clinical benefits of PRP appear to result from enhanced differentiation of tumor cells, which inhibits proliferation and consequently reduces their ability to invade and metastasize.
Specifically, the research showed that pro-enzymes:
| ● | induce a dose-dependent inhibition of cell growth, triggering apoptosis and cell necrosis; | |
| ● | enhance expression of epithelial markers, such as E-cadherin and β-catenin; | |
| ● | decrease expression of EMT transcription factors responsible for coding specific gene sequences from DNA, associated with TGF-β cell signaling pathways; and | |
| ● | induce malignant cells to differentiate to benign forms. |
Once activated, pro-enzymes influence the micro-immune environment around the cell, altering a number of pathways critical for supporting cancer cell growth, invasion and metastasis. This includes interacting with proteinases and cell signaling pathways in the extracellular matrix, whilst also interacting directly with cell surface proteins that effect the internal pathways of the cancer cell, triggering re-expression of epithelial markers, reducing important EMT markers, and inducing a series of cellular activities which alters the cancer cell’s morphology (structure) from a malignant to a benign state.
Planned Clinical Development
PRP recently completed preclinical development. A First-In-Human (FIH), Phase Ib study in patients with advanced solid tumors, evaluating the safety, pharmacokinetics and anti-tumor efficacy of PRP is planned to commence in 2019 calendar year subject to us receiving adequate financing, and is hoped to be completed within twelve months. The study will be an open-label, multicenter, non-comparative study of PRP administered at increasing dose levels, with once daily intravenous injections over a 28-day cycle, with at least 20 and up to 40 patients enrolled.
The Phase Ib study is planned to be followed by two open Phase IIa studies evaluating the safety, pharmacokinetics and anti-tumor efficacy of PRP administered intravenously to patients with locally advanced or metastatic pancreatic adenocarcinoma, or to patients with advanced epithelial ovarian cancer who have failed prior anti-cancer therapy regimen. These studies are envisioned to start in parallel, shortly after the FIH Phase IIa study, and are hoped to be finalized in 2021. Both studies will be open, multicenter phase II studies measuring overall survival of patients having received once daily intravenous administrations of PRP.
Preclinical Development
We have extensive in vitro and in vivo studies demonstrating the anti-tumor efficacy of a novel pro-enzyme formulation consisting of a combination of trypsinogen and chymotrypsinogen in a synergistic ratio. The preclinical work was undertaken in collaboration with our contract research organization, vivoPharm, in both Melbourne, Australia and Hummelstown, PA, United States, together with universities we partnered with, including the Biopathology and Regenerative Medicine Institute, Center for Biomedical Research, at the University of Granada in Granada, Spain, and the Department of Health Sciences at the University of Jaén in Jaén, Spain. We funded both vivoPharm and the universities to carry out this research and retained the intellectual property rights within the field relating to any discoveries based on the mechanism of action and anti-tumor effects of the pro-enzymes.
| 42 |
The following preclinical development activities have been undertaken to date:
| ● | We tested the anti-proliferative effects of trypsinogen and chymotrypsinogen in 24 cancer cell lines and determined a synergistic ratio of 1:6, which we used to formulate PRP; | |
| ● | We evaluated the in vitro anti-angiogenic effects of PRP, by soft-agar formation assay, and in vivo using the AngioChamber™ assay, which is based on the normal physiological process of wound healing, to promote fibrous capsule formation around an implanted growth factor-releasing Teflon chamber; | |
| ● | To analyze the anti-metastatic effects of pro-enzymes, we studied the effects of PRP in cell invasion, cell migration, and in the modulation of EMT related genes in pancreatic and ovarian cancer cells; and | |
| ● | We also performed in vivo a pharmacokinetic study and assessed the anti-tumor efficacy of PRP in murine cancer models. To accomplish this, we treated mice that were orthotopically inoculated with A2780 human ovarian cancer cells, or with Pan02 mouse pancreatic tumor cells, with PRP. |
Determination of Optimal Pro-enzyme Ratio
In this study, we determined first the half-maximal inhibitory concentrations (IC50) trypsinogen and chymotrypsinogen to measure their effect as single test articles in an extended panel of 24 human cancer cell lines. The IC50 values of trypsinogen ranged from 2.5 to 17.5 mg/ml and from 1.4 to 25.2 mg/ml for chymotrypsinogen. The IC50 values of trypsinogen were the basis for the calculation of concentration ratios for the combination of trypsinogen and chymotrypsinogen at 1:1, 1:2, 1:4, 1:6, 1:8, and 1:10. At these ratios, the growth inhibitory properties of the combination were evaluated in 24 cancer cell lines. Based on the coefficient of drug interaction (CDI) values, the combination of trypsinogen and chymotrypsinogen demonstrated greater growth inhibition at ratios of 1:4, 1:6, and 1:8, compared to the 1:1 ratio in most cell lines tested. Finally, a ratio of trypsinogen to chymotrypsinogen of 1:6 was determined to be the optimal formulation and used for later experiments.
Anti-angiogenic efficacy of pancreatic proenzyme formulation
To determine whether PRP affects angiogenesis, we used a soft-agar tube formation assay. Dispersed human umbilical vein endothelial cells (HUVEC) organized into clusters after three hours and began to form tube-like structures after five hours that were clearly evident after 24 hours. In contrast, PRP treated HUVECs presented a marked reduction in the number and length of closed capillary tubes in a concentration dependent manner, with a total disappearance of the structures after treatment with trypsinogen to chymotrypsinogen (T/C) 0.07/0.42 mg/ml. To assess if the inhibition of the tubule-like structures formation could be due to cell death caused by PRP treatment, CellTracker Green/CMFDA staining was used to identify viable cells. Both control and PRP treated cells showed green staining, indicating that the inhibition of cellular cords was independent from cell viability. Furthermore, quantification of the number of capillary-like structures at different areas of the cell revealed a dramatic and significant difference between the number of structures formed by non-treated cells when compared with PRP-treated cells (p <0.01 vs. Control).
| 43 |
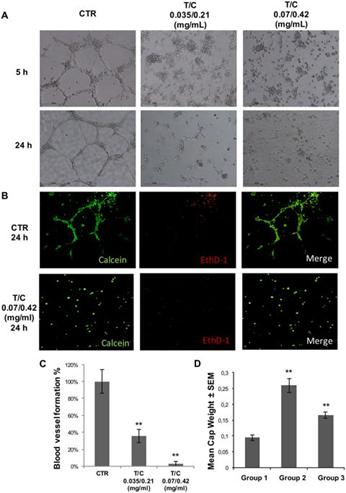
The anti-angiogenic effect of PRP was additionally investigated in vivo using the AngioChamber™ assay, a model used to assess the efficacy of anti-angiogenic treatments by measuring fibrous capsule formation in mice. In this assay the inclusion of basic fibroblast growth factor (bFGF) in the chamber supports the induction of blood vessels development and formation of a fibrous capsule. AngioChambers™ were excised from all post-mortem mice on the termination day, 24 hours following final treatment (Day 5).
The results show that fibrous capsule formation was significantly greater in the vehicle control group with bFGF captured in the chamber (Group 2, Induction Control) than in the vehicle control group without bFGF loaded into the chamber (Group 1, Baseline Control) (p<0.05) indicating that bFGF adequately and significantly stimulated capsule formation. Furthermore, treatment with PRP (Group 3) resulted in a significant reduction in angiogenesis compared to the induction control (Group 2), as indicated by the difference in capsule weight (p < 0.05) with a 57% of fibrous capsule formation inhibition. Thus, PRP inhibits fibrous capsule formation showing significant in vivo anti-angiogenic effects.
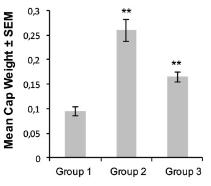
Anti-invasion, anti-migration and anti-EMT effect of PRP
To analyze the in vitro anti-metastatic effect of the pro-enzyme treatment, we studied the effect of PRP in cell invasion, cell migration and the modulation of EMT related genes in cancer cells. First, to evaluate the effect of PRP on cell migration, a key event in carcinogenesis, we performed a wound-healing assay on human pancreatic BxPC3 and human ovarian A2780 cells. Migration is defined as the directed movement of cells on a substrate such as plastic plates occurring on 2D surfaces.
| 44 |
Results show that non-treated cells migrated faster to close the gap of a scratch in the cell monolayer than PRP treated cells. PRP significantly reduced cell migration of pancreatic BxPC3 cells and compared with control cells even enhanced the width of the wound.
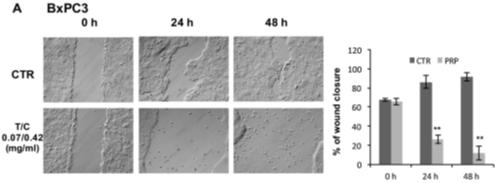
Although the A2780 ovarian tumor cell line does not grow forming a homogeneous monolayer like BxPC3, it can be observed that PRP treatment significantly reduces the ability of the ovarian cells to migrate. Data showed significant cell migration inhibition after 24 hours and 48 hours of treatment with PRP compared to control cells.
Secondly, we tested the inhibitory effect of the pro-enzyme formulation on cell invasion of colon and pancreatic tumor cells. Invasion is defined as cell movement through an extracellular 3D matrix. The principle of this assay is based on two medium containing chambers separated by a porous membrane through which cells transmigrate. Here, we tested different concentrations of PRP on MIA PaCa-2 pancreatic and HCT-15 colon human cancer cell lines. PRP showed a marked and significantly dose-dependent inhibition of invasion in both cell lines. Total inhibition of cell migration was achieved from PRP concentrations of T/C 0.015/0.093 mg/ml and so on with the other increasing concentrations tested.
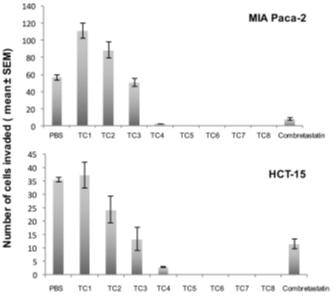
Finally, to investigate whether the exposure of PRP has a potential regulation in the transcriptional machinery that drives EMT in cancer cells, expression of EMT genes were studied in BxPC3 pancreatic and A2780 ovarian human cancer cells. EMT markers in both BxPC3 and A2780 cells were affected by PRP treatment at T/C 0.07/0.42 mg/ml. Results show that PRP treatment increased the expression of E-Cadherin (0.4 fold) (p < 0.05), whilst reduced the expressions of N-cadherin, Slug and vimentin (0.9, 0.5 and 0.6 fold, respectively) (p < 0.01) in BxPC3 cells.
In addition, PRP significantly up-regulated the expression of E-Cadherin (0.9 fold) (p < 0.01) and significantly down-regulated the expression of N-cadherin and Slug (0.4 and 0.6 fold, respectively) (p < 0.01) and induced a slight, but not significant, decrease of vimentin expression in A2780 cells.
| 45 |
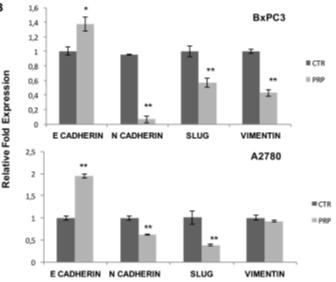
PRP pharmacokinetic study
To evaluate the pharmacokinetics and organ distribution of trypsinogen and chymotrypsinogen, non-tumor bearing female athymic Nude-Foxn1nu mice were treated with IRDye® 800CW labeled trypsinogen (5 mg/kg) plus unlabeled trypsinogen (50 mg/kg), or IRDye® 800 CW labeled chymotrypsinogen (7 mg/kg) plus unlabeled Chymotrypsinogen (300 mg/kg). Animals were euthanized at specified time-points post-dose and plasma along with organ homogenates was prepared, then imaged via IVIS imaging system.
Fluorescence was measured in organ homogenates. Mice treated with labeled T, presented a fluorescence peak in all organs between 15 minutes and 2 hours post-dose. While mice treated with labeled C showed the maximum fluorescent emission between 15 minutes and 6 hours post-dose. For both highest readings were observed in the kidneys and liver. Maximum levels of both IRDye® 800CW labeled trypsinogen and chymotrypsinogen A in mouse plasma occurred at 15 minutes post dose (7.5 and 72.2 ìg/ml, respectively). Levels of both IRDye® 800CW labeled proenzymes decreased rapidly after this time.
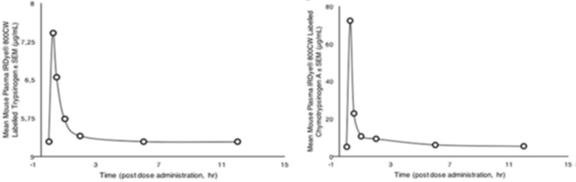
Anti-tumor efficacy of PRP in orthotropic mice models
The effect of the pro-enzyme formulation PRP at different doses on tumor weight in orthotopically implanted pancreatic and ovary tumors was assessed. In the pancreatic tumor control group, there was significant (*P < 0.05) reduction in mean tumor weight in animals treated for 26 days with trypsinogen/chymotrypsinogen at 83.3/500 mg/kg (30.2 mg; 85.9% inhibition) compared with control (PBS; 214.8 mg), but not between trypsinogen/chymotrypsinogen at 27.5/165 mg/kg (196.5 mg; 8.5 % inhibition) and the control (as shown in the figure below). Furthermore, ovary tumor-bearing mice (as shown in the figure below) showed a significant (p < 0.05) reduction in mean tumor weight in animals treated for 21 days with two different doses of trypsinogen/chymotrypsinogen, 9.1/54 mg/kg and 27.5/165 mg/kg, compared with control (PBS). The mean weight of control group tumors was 2062.2 mg while the treated groups presented a mean tumor weight of 1074.2 mg and 957.3 respectively, ranging in a 50% tumor inhibition (52% - 46%).
| 46 |
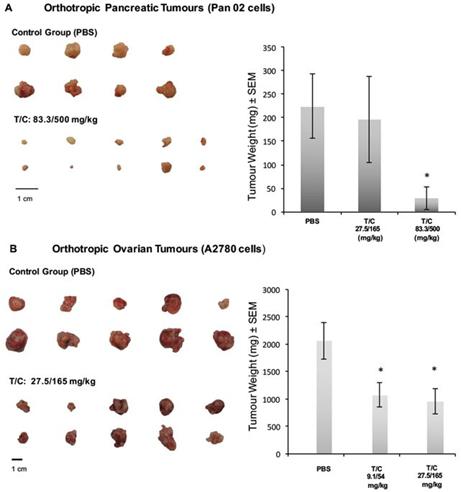
The PRP Formulation
Oral pancreatic enzymes have been administered previously in a variety of circumstances and are in current clinical use in conditions where the pancreas is unable to produce sufficient enzymes for the digestion of food. A number of oral pancreatic enzyme products are presently approved in the U.S. for use in patients who do not produce enough pancreatic enzymes. Approved pancreatic enzyme products include Pancreaze™ from Johnson & Johnson, CREON® from Abbott Laboratories, and ULTRASE® from Axcan Pharma US.
PRP is a combination of two pro-enzymes, trypsinogen and chymotrypsinogen, specifically formulated within a specific ratio (1:6, as described above) designed to synergistically enhance their anti-cancer effects based on the mechanism of action. Patent protection for PRP has been secured in multiple jurisdictions, including the United States, and continues to be sought for similar compositions and mechanisms of action.
Oral enzymes have also been investigated previously for the treatment of cancer and, while generating encouraging results, their widespread use has been hampered by the very large quantities that have been considered necessary for effective treatment – 130 or more tablets per day. The high dose used with oral delivery is considered necessary due to the oral enzymes being broken down in the stomach and duodenum, the first part of the small intestine and very little actually being absorbed into the general circulation. By administering a pro-enzyme parenterally, and using a specific pro-enzyme formulation, the normal breakdown of the enzymes when taken orally is avoided and the drug can potentially be absorbed into the general circulation intact. It is also suggested that pro-enzymes are resistant to inactivation by numerous protein digesting enzymes, like serpins, which are circulating in the blood. Together with our scientific consultants, we believe that the development of a parenteral pro-enzyme formulation will lead to improved efficacy in the treatment of cancer compared with oral enzyme preparations, and will substantially reduce the dose in comparison to that used previously for oral enzyme therapy for the treatment of cancer.
| 47 |
Target Indications
The management of cancer differs widely, with a multitude of factors impacting the choice of treatment strategy. Some of those factors include:
| ● | the type of tumor, usually defined by the tissue in the body from which it originated; | |
| ● | the extent to which it has spread beyond its original location; | |
| ● | the availability of treatments, driven by multiple factors including cost, drugs approved, local availability of suitable facilities, etc.; | |
| ● | regional and geographic differences; | |
| ● | whether the primary tumor is amenable to surgery, either as a potentially curative procedure, or as a palliative one; and | |
| ● | the balance between potential risks and potential benefits from the various treatments and, probably most importantly, the patient’s wishes. |
For many patients with solid cancers, such as breast, ovarian, colorectal, lung and pancreatic cancer, surgery is frequently the first treatment option, often followed by first line chemotherapy with or without radiotherapy. While hopefully such procedures are curative, in many instances the tumor returns, and second line treatment strategies are chosen in an effort to achieve a degree of control over the tumor. In most instances, the benefit is temporary, and eventually the point is reached where the patient’s tumor either fails to adequately respond to treatment, or the treatment has unacceptable toxicity which severely limits its usefulness.
Should the planned Phase I, II and III clinical trials confirm the efficacy of PRP, along with the favorable safety and tolerability profile suggested by pre-clinical studies conducted to date, we believe our product will have utility in a number of clinical situations including:
| ● | in the early stage management of solid tumors, most likely as part of a multi-pronged treatment strategy in combination with existing therapeutic interventions; | |
| ● | as a product that can be administered long term for patients following standard treatment approaches, such as surgery, or chemotherapy, in order to prevent or delay recurrence; and | |
| ● | as a preventative measure for patients at risk of developing cancer based on genetic screening. |
In the near term as part of our planned Phase I, II and III clinical trials, we plan to target patients with solid tumors, most likely ovarian and pancreatic, for whom other treatment options have been exhausted. This is a common approach by which most new drugs for cancer are initially tested. Once efficacy and safety has been demonstrated in this patient population, exploration of the potential utility of the drug in earlier stage disease can be undertaken, together with investigation of the drug’s utility in other types of cancers, such as gastro-esophageal tumors, colon or rectal carcinoma might be conducted. A Phase II study in a back-up indication, such as advanced therapy refractant prostate cancer will also be considered. This indication is based on positive preclinical pharmacology studies.
Development Strategy
Our goal is to undertake early stage clinical development of PRP through to a significant value inflection point, where the commercial attractiveness of a drug in development, together with a greater likelihood of achieving market authorization, may attract potential interest from licensees seeking to acquire new products. Such value inflection points in the context of cancer drugs are typically at the point where formal, controlled clinical trials have demonstrated either ‘efficacy’ or ‘proof of concept’ – typically meaning that there is controlled clinical trial evidence that the drug is effective in the proposed target patient population, has an acceptable safety profile, and is suitable for further development. From a ‘big picture’ perspective, it is our intention to progress the development of our technology through the completion of our planned Phase IIa clinical trials and then to seek a licensee for further development beyond that point.
| 48 |
As part of that commercial strategy, we will:
| ● | continue research and development to build our existing intellectual property portfolio, and to seek new, patentable discoveries; | |
| ● | seek to ensure all product development is undertaken in a manner that makes its products approvable in the major pharmaceutical markets, including the U.S., Europe, the UK, Australia and Japan; | |
| ● | aggressively pursue the protection of our technology through all means possible, including patents in all major jurisdictions, and potentially trade secrets; and | |
| ● | make strategic acquisitions to acquire new companies that have products or services that complement our future goals. |
Development Plan and Milestones
PRP
We plan to progress PRP down a conventional early stage clinical development pathway for:
| ● | regulatory and/or ethics approval to conduct a Phase Ib study, and submit it with the applicable government agency for approval; and | |
| ● | Phase IIa multiple escalating dose studies to investigate the safety, tolerability, and pharmacokinetics of PRP administered intravenously to patients. |
We are currently evaluating Australia, UK and Europe as the potential destination where we may commence the Phase Ib trial. In particular, we are closely evaluating Australia because of its research and development tax incentives, as well as a simplified regulatory environment. As part of such incentives, eligible companies conducting clinical trials in Australia may receive up to 43.5% “cash-back” benefit in the form of a refund of their qualified research and development costs and expenses. We are evaluating all options to conduct our planned clinical trials in the most cost-efficient manner, while striving to minimize dilution to our stockholders.
We anticipate reaching the Phase IIa proof of concept milestone in approximately three to four years, subject to regulatory approval in Europe, and the results from our research and development and licensing activities.
Our overhead and expenses are likely to increase from its current level as PRP progresses down the development pathway. This increase will be driven by the need to increase our internal resources in order to effectively manage our research and development activities.
Anticipated timelines
Commencing in the 2019 calendar year, we intend to initiate a Phase Ib study in advanced cancer patients with solid tumors and the anticipated costs will be approximately $2,500,000.
Financial Objective
Multiple factors, many of which are outside of our control, can impact our ability to achieve our target objectives within the planned time and budgetary constraints. Subject to these caveats, our objective is to complete our planned Phase IIa study for PRP within the proposed budget.
Corporate Strategy
We primarily outsource services, skills and expertise to third parties as required to achieve our scientific and corporate objectives. As the business grows and gains more personnel, outsourcing will continue to be the preferred model, where fixed and variable costs are carefully managed on a project-by-project basis. This means our research and development activities are carried out by third parties. Additional third parties with specific expertise in research, compound screening and manufacturing (including raw material suppliers) have been contracted as required.
Our initial focus is to organize, coordinate and finance the various parts of our drug development pipeline. New personnel will be carefully introduced into our Company over a period of time as our research and development activities expand. They will have specific expertise in product development, manufacture and formulation, regulatory affairs, toxicology, clinical operations and business development (including intellectual property management, licensing and other corporate activities).
| 49 |
In the first instance, additional clinical management and development expertise is likely to be required for our lead product. Therefore, we anticipate an increase in employees in order to effectively manage our contractors as the projects progress down the development pathway.
This outsourcing strategy is common in the biotechnology sector, and is an efficient way to obtain access to the necessary skills required to progress a project, in particular as the required skills change as the project progresses from discovery, through manufacturing and non-clinical development and into clinical trials. We anticipate that we will continue to use this model, thereby retaining the flexibility to contract in the appropriate resource as and when required.
We intend to seek and identify potential licensing partners for our product candidates as they progress through the various development stages, reaching certain milestones and value inflection points. If a suitable licensee is identified, a potential licensing deal could consist of payments for certain milestones, plus royalties from future sales if the product is able to receive approval from the relevant regulatory authorities where future product sales are targeted. We intend to seek and identify potential licensees based on the initial efficacy data from Phase II clinical trials. To accomplish this objective, we have commenced discussions with potential partners in our current preclinical phase of development.
As part of our overall expansion strategy, from time to time, we investigate potential intellectual property acquisition opportunities to expand our product portfolio. While our initial focus is on the development of PRP as the lead product candidate, potential product candidates may also be considered for future preclinical and clinical development. These potential opportunities have arisen from other research and development organizations, which either own existing intellectual property or are currently developing new intellectual property, which may be of interest to us. These opportunities are possible new cancer treatments that are potentially less toxic than existing treatment approaches and are able to fill an existing gap in the treatment process, such as a systemic de-bulking method which could reduce the size and threat of metastases to a more manageable level for late stage cancer patients. We believe these potential treatment approaches will be complementary to existing treatment regimens and our existing product candidate, PRP. No formal approaches have been made at this stage and it is unknown whether we will engage in this discussion in the near future. However, we remain hopeful that as PRP progresses further down the development pathway, future opportunities may arise to use the expertise of our management and scientific personnel for future prospective research and development projects.
Current Operations
We are at a pre-revenue stage. We do not know when, if ever, we will be able to commercialize our products and begin generating revenue. We are focusing our efforts on organizing, coordinating and financing the various aspects of the drug research and development program outlined earlier in this document. In order to commercialize our products, we must complete preclinical development, Phase Ib, IIa and IIb clinical trials in Europe, the U.S., United Kingdom, Australia or elsewhere, and satisfy the applicable regulatory authority that PRP is safe and effective. If the results from the Phase II trials are convincing, we will seek conditional approval from the regulatory authorities sooner. Therefore, we estimate that this will take approximately three to four years if we seek conditional approval, or up to seven years if we determine that Phase III trials are needed. As described previously, when we advance our development projects sufficiently down the development pathway and achieve a major increase in value, such as obtaining interim efficacy data from Phase II clinical trials, we will seek a suitable licensing partner to complete the remaining development activities, obtain regulatory approval and market the product.
Current Therapies/Drugs Available
We are developing a therapeutic solution for the treatment of patients with advanced stages of cancer targeting solid tumors, which is cancer that originates in organs or tissues other than bone marrow or the lymph system. Common cancer types classified as solid tumors include lung, colorectal, ovarian cancer, pancreatic cancer and liver cancers. In each of these indications, there is a large market opportunity to capitalize on the limitations of current therapies.
Current therapeutic options for the treatment of cancer offer, at most, a few months of extra life or tumor stabilization. Some experts believe that drugs that kill most tumor cells do not affect cancer stem cells, which can regenerate the tumor (e.g. chemotherapy). Studies are revealing the genetic changes in cells that cause cancer and spur its growth. This research is providing scientific researchers with many potential targets for drugs. Tumor cells, however, can develop resistance to drugs.
Limitations of Current Therapies
PRP was developed because of the limitation of current cancer therapies. While surgery is often safe and effective for early stage cancer, many standard therapies for late stage cancer urgently need improvement; current treatments generally provide modest benefits, and frequently cause significant adverse effects. Our focus is to provide oncologists and their patients with therapies for metastatic cancer which are more effective than current therapies, and which have a substantially reduced side effect profile.
| 50 |
While progress has been made within the oncology sector in developing new treatments, the overall cancer death rate has only improved by 7% over the last 30 years. Most of these new treatments have some limitations, such as:
| ● | significant toxic effects; | |
| ● | expense; and | |
| ● | limited survival benefits. |
We believe that our treatment will provide a competitive advantage over the following treatments:
| ● | Chemotherapeutics: Side effects from chemotherapy can include pain, diarrhea, constipation, mouth sores, hair loss, nausea and vomiting, as well as blood-related side effects, which may include a low cell count of infection fighting white blood cells (neutropenia), low red blood cell count (anemia), and low platelet count (thrombocytopenia). Our goal is to demonstrate that our treatment will be more effective than chemotherapeutic and hormonal therapies with fewer side effects. | |
| ● | Targeted therapies: The most common type is multi-targeted kinase inhibitors (molecules which inhibit a specific class of enzymes called kinases). Common side effects include fatigue, rash, hand–foot reaction, diarrhea, hypertension and dyspnoea (shortness of breath). Furthermore, tyrosine kinases inhibited by these drugs appear to develop resistance to inhibitors. While the clinical findings with PRP are early and subject to confirmation in future clinical trials, no evidence has yet been observed of the development of resistance by the cancer to PRP. | |
| ● | Monoclonal antibodies: Development of monoclonal antibodies is often difficult due to safety concerns. Side effects that are most common include skin and gastro-intestinal toxicities. For example, several serious side effects from Avastin, an anti-angiogenic cancer drug, include gastrointestinal perforation and dehiscence (e.g. rupture of the bowel), severe hypertension (often requiring emergency treatment) and nephrotic syndrome (protein leakage into the urine). Antibody therapy can be applied to various cancer types, but can also be limited to certain genetic sub populations in many instances. | |
| ● | Immunotherapy: There is a long history of attempts to develop therapeutic cancer vaccines to stimulate the body’s own immune system to attack cancer cells. While these products generally do not have the poor safety profile of standard therapeutic approaches, only a relatively small number of them are FDA-approved and available as compared to the number of patients diagnosed with cancer. Furthermore, only a relatively small number of the patient population is eligible to receive and subsequently respond to treatment, as defined by preventing tumor growth. |
License Agreements
We previously sponsored a collaborative research project at Bath University to investigate the cellular and molecular mechanisms underlying the potential clinical approach of our proprietary pro-enzyme formulation. As a result of this undertaking, we entered into a Commercialization Agreement with University of Bath (UK), dated November 12, 2009 (the “Commercialization Agreement”), where, initially, we held an exclusive license with Bath University, and where we and Bath University co-owned the intellectual property relating to our pro-enzyme formulations. The Commercialization Agreement originally provided for Bath University to assign the Patents (as defined therein) to Propanc in certain specified circumstances, such as successful completion of a clinical trial and commencement of a Phase II (Proof of Concept) clinical trial.
On June 14, 2012, Propanc and Bath University agreed to an earlier assignment to us of the patents pursuant to an Assignment and Amendment Deed, on the proviso that Bath University retains certain rights arising from the Commercialization Agreement, as follows:
| ● | Bath University reserves for itself (and its employees and students and permitted academic sub-licensees with respect to research use) the non-exclusive, irrevocable, worldwide, royalty free right to use the patents for research use; | |
| ● | The publication rights of Bath University specified in the contract relating to the original research made between the parties with an effective date of July 18, 2008 shall continue in force; | |
| ● | Propanc shall pay to Bath University a royalty of two percent of any and all net revenues; | |
| ● | Propanc shall use all reasonable endeavors to develop and commercially exploit the patents for the mutual benefit of Bath University and Propanc to the maximum extent throughout the covered territory and in any additional territory and to obtain, maintain and/or renew any licenses or authorizations that are necessary to enable such development and commercial exploitation. Without prejudice to the generality of the foregoing, Propanc shall comply with all relevant regulatory requirements in respect of its sponsoring and/or performing clinical trials in humans involving the administration of a product or materials within a claim of the patents; and | |
| ● | Propanc shall take out with a reputable insurance company and maintain liability insurance coverage prior to the first human trials. |
| 51 |
In consideration of such assignment, we agreed to pay royalties of 2% of net revenues to Bath University. Additionally, we agreed to pay 5% of each and every license agreement subscribed for. The contract is cancellable at any time by either party. To date, no amounts are owed under the agreement.
We continue to learn the properties of pro-enzymes with the long-term aim of screening new compounds for development. We anticipate engaging in future discussions with several technology companies who are progressing new developments in the oncology field as potential additions to our product line. Initially targeting the oncology sector, our focus is to identify and develop novel treatments that are highly effective targeted therapies, with few side effects as a result of toxicity to healthy cells.
Intellectual Property
We have filed multiple patent applications relating to our lead product, PRP. The first application was filed in October 2010 in each of the countries listed in the table below. This application has been granted and remains in force in the United States, Belgium, Czech Republic, Denmark, France, Germany, Ireland, Italy, Netherlands, Portugal, Spain, Sweden, Switzerland, Liechtenstein, Turkey, United Kingdom, Australia, China, Japan, Indonesia, Israel, New Zealand, Singapore, Malaysia, South Africa, Mexico, Republic of Korea and India. In Brazil and Canada, the patent application remains under examination.
In 2016 and 2017 we filed other patent applications, as indicated below. Three applications were filed under the PCT. The PCT assists applicants in seeking patent protection by filing one international patent application under the PCT, which allows the applicants to seek protection for an invention in over 150 countries. Once national or regional applications are filed, the application is placed under the control of the national or regional patent offices, as applicable, in what is called the national or regional phase. One PCT application, filed in November 2016, entered the national phase in July 2018 in each of the countries listed in the table below. A second application filed in January 2017 entered the national phase commencing July 2018. A third application entered the national phase in October 2018.
| No. | Title | Country | Case Status | Date Filed | ||||
| 1. | A pharmaceutical composition for treating cancer comprising trypsinogen and/or chymotrypsinogen and an active agent selected from a selenium compound, a vanilloid compound and a cytoplasmic reduction agent. | USA, Belgium, Czech Republic, Denmark, France, Germany, Ireland, Italy, Netherlands, Portugal, Spain, Sweden, Switzerland, Liechtenstein, Turkey, United Kingdom, Australia, China, Japan, Indonesia, Israel, New Zealand, Malaysia, Singapore, Malaysia South Africa, Mexico, Republic of Korea and India | Granted | Oct-22-2010 | ||||
| Brazil and Canada | Under Examination
Divisional applications filed and under examination in USA, Mexico and China |
|||||||
| 2. | Proenzyme composition | Australia, Canada, China, Europe, Hong Kong, India, Indonesia, Israel, Japan, Malaysia, New Zealand, Singapore, South Africa and USA | Application filed and pending | Nov-11-2016 | ||||
| 3. | Cancer Treatment | Australia, Canada, China, Europe, Hong Kong, Israel, Japan, Malaysia, New Zealand, Singapore and USA | Application filed and pending | Jan-27-2017 | ||||
| 4. | Composition of proenzymes for cancer treatment | Australia, China, Europe, Japan, and USA | Application filed and pending | Apr-12-2017 |
| 52 |
Further patent applications are expected to be filed to capture and protect additional patentable subject matter based on our field of technology relating to pharmaceutical compositions of proenzymes for treating cancer.
The basis of our intellectual property protection will be built around the following elements:
| ● | Method of use: Understanding the mechanism of action of the PRP pro-enzyme formulations, enabling the identification of new molecular targets, potential new therapeutic compounds and identification of new formulations that are adapted to enhance activity. | |
| ● | Formulation: We have developed an enhanced formulation containing the pro-enzyme trypsinogen in combination with at least one of two types of identified compounds considered effective for providing synergistic enhancement of the pro-enzyme based formulations. A patentability assessment, based on an international prior art search, has indicated that strong potential exists for successfully obtaining patent claims covering the formulation. | |
| ● | Composition of Matter: Synthetic recombinant proteins designed to improve the quality, safety and performance of pro-enzymes used in the proposed formulations form part of the research and development program. |
Regulatory Issues
United States
Government oversight of the pharmaceutical industry is usually classified into pre-approval and post-approval categories. Most of the therapeutically significant innovative products marketed today are the subject of New Drug Applications (“NDA”). Preapproval activities, based on these detailed applications, are used to assure the product is safe and effective before marketing. In the United States, The Center for Drug Evaluation and Research (“CDER”), is the FDA organization responsible for over-the-counter and prescription drugs, including most biological therapeutics, and generic drugs.
Before approval, the FDA may inspect and audit the development facilities, planned production facilities, clinical trials, institutional review boards and laboratory facilities in which the product was tested in animals. After the product is approved and marketed, the FDA uses different mechanisms for assuring that firms adhere to the terms and conditions of approval described in the application and that the product is manufactured in a consistent and controlled manner. This is done by periodic unannounced inspections of production and quality control facilities by FDA’s field investigators and analysts.
Federal Food, Drug and Cosmetic Act and Public Health Service Act
Prescription drug and biologic products are subject to extensive pre- and post-market regulation by the FDA, including regulations that govern the testing, manufacturing, safety, efficacy, labelling, storage, record keeping, advertising and promotion of such products under the Federal Food, Drug and Cosmetic Act, the Public Health Service Act, and their implementing regulations. The process of obtaining FDA approval and achieving and maintaining compliance with applicable laws and regulations requires the expenditure of substantial time and financial resources. Failure to comply with applicable FDA or other requirements may result in refusal to approve pending applications, a clinical hold, warning letters, civil or criminal penalties, recall or seizure of products, partial or total suspension of production or withdrawal of the product from the market. FDA approval is required before any new drug or biologic, including a new use of a previously approved drug, can be marketed in the United States. All applications for FDA approval must contain, among other things, information relating to safety and efficacy, stability, manufacturing, processing, packaging, labelling and quality control.
New Drug Applications (“NDAs”)
The FDA’s NDA approval process generally involves:
| ● | Completion of preclinical laboratory and animal testing in compliance with the FDA’s good laboratory practice, or GLP, regulations; | |
| ● | Submission to the FDA of an investigational new drug (“IND”) application for human clinical testing, which must become effective before human clinical trials may begin in the United States; | |
| ● | Performance of adequate and well-controlled human clinical trials to establish the safety, purity and potency of the proposed product for each intended use; |
| 53 |
| ● | Satisfactory completion of an FDA pre-approval inspection of the facility or facilities at which the product is manufactured to assess compliance with the FDA’s “current good manufacturing practice” (“CGMP”) regulations; and | |
| ● | Submission to and approval by the FDA of a NDA. |
The preclinical and clinical testing and approval process requires substantial time, effort and financial resources, and we cannot guarantee that any approvals for our product candidates will be granted on a timely basis, if at all. Preclinical tests include laboratory evaluation of toxicity and immunogenicity in animals. The results of preclinical tests, together with manufacturing information and analytical data, are submitted as part of an IND application to the FDA. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA raises concerns or questions about the conduct of the clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before clinical trials can begin. Our submission of an IND may not result in FDA authorization to commence clinical trials. A separate submission to an existing IND must also be made for each successive clinical trial conducted during product development. Further, an independent institutional review board (“IRB”) covering each medical center proposing to conduct clinical trials must review and approve the plan for any clinical trial before it commences at that center and it must monitor the study until completed. The FDA, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk. Clinical testing also must satisfy extensive “good clinical practice” (“GCP”) regulations, which include requirements that all research subjects provide informed consent and that all clinical studies be conducted under the supervision of one or more qualified investigators.
For purposes of an NDA submission and approval, human clinical trials are typically conducted in the following sequential phases, which may overlap:
| ● | Phase I: Initially conducted in a limited population to test the product candidate for safety and dose tolerance; | |
| ● | Phase II: Generally conducted in a limited patient population to identify possible adverse effects and safety risks, to determine the initial efficacy of the product for specific targeted indications and to determine optimal dosage. A Phase IIa trial is a non-pivotal, exploratory study that assesses biological activity as its primary endpoint. A Phase IIb trial is designed as a definite dose finding study with efficacy as the primary endpoint. Multiple Phase II clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more extensive Phase III clinical trials; | |
| ● | Phase III: Commonly referred to as pivotal studies. When Phase II evaluations demonstrate that a dose range of the product is effective and has an acceptable safety profile, Phase III clinical trials are undertaken in large patient populations to further evaluate dosage, to provide substantial evidence of clinical efficacy and to further test for safety in an expanded and diverse patient population at multiple, geographically-dispersed clinical trial sites. Generally, replicate evidence of safety and effectiveness needs to be demonstrated in two adequate and well-controlled Phase III clinical trials of a product candidate for a specific indication. These studies are intended to establish the overall risk/benefit ratio of the product and provide adequate basis for product labelling; and | |
| ● | Phase IV: In some cases, the FDA may condition approval of a NDA on the sponsor’s agreement to conduct additional clinical trials to further assess the product’s safety, purity and potency after NDA approval. Such post-approval trials are typically referred to as Phase IV clinical trials. |
Progress reports detailing the results of the clinical studies must be submitted at least annually to the FDA and safety reports must be submitted to the FDA and the investigators for serious and unexpected adverse events. Concurrent with clinical studies, sponsors usually complete additional animal studies and must also develop additional information about the product and finalize a process for manufacturing the product in commercial quantities in accordance with CGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final product. Moreover, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
The results of product development, preclinical studies and clinical trials, along with the aforementioned manufacturing information, are submitted to the FDA as part of a NDA. NDA’s must also contain extensive manufacturing information. Under the Prescription Drug User Fee Act (“PDUFA”), the FDA agrees to specific goals for NDA review time through a two-tiered classification system, Standard Review and Priority Review. Standard Review is applied to products that offer at most, only minor improvement over existing marketed therapies. Standard Review NDAs have a goal of being completed within a ten-month timeframe, although a review can take significantly longer. A Priority Review designation is given to products that offer major advances in treatment, or provide a treatment where no adequate therapy exists. A Priority Review takes the FDA six months to review a NDA. It is likely that our product candidates will be granted Standard Reviews. The review process is often significantly extended by FDA requests for additional information or clarification. The FDA may refer the application to an advisory committee for review, evaluation and recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations.
| 54 |
The FDA may deny approval of a NDA if the applicable regulatory criteria are not satisfied, or it may require additional clinical data or additional pivotal Phase III clinical trials. Even if such data is submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Data from clinical trials is not always conclusive and the FDA may interpret data differently than Propanc. Once issued, product approval may be withdrawn by the FDA if ongoing regulatory requirements are not met or if safety problems occur after the product reaches the market. In addition, the FDA may require testing, including Phase IV clinical trials, Risk Evaluation and Mitigation Strategies (“REMS”), and surveillance programs to monitor the effect of approved products that have been commercialized, and the FDA has the power to prevent or limit further marketing of a product based on the results of these post-marketing programs. Products may be marketed only for the approved indications and in accordance with the provisions of the approved label. Further, if there are any modifications to the drug, including changes in indications, labelling or manufacturing processes or facilities, approval of a new or supplemental NDA may be required, which may involve conducting additional preclinical studies and clinical trials.
Other U.S. Regulatory Requirements
After approval, products are subject to extensive continuing regulation by the FDA, which include company obligations to manufacture products in accordance with GMP, maintain and provide to the FDA updated safety and efficacy information, report adverse experiences with the product, keep certain records, submit periodic reports, obtain FDA approval of certain manufacturing or labeling changes and comply with FDA promotion and advertising requirements and restrictions. Failure to meet these obligations can result in various adverse consequences, both voluntary and FDA-imposed, including product recalls, withdrawal of approval, restrictions on marketing and the imposition of civil fines and criminal penalties. In addition, later discovery of previously unknown safety or efficacy issues may result in restrictions on the product, manufacturer or NDA holder.
Propanc, and any manufacturers of our products, are required to comply with applicable FDA manufacturing requirements contained in the FDA’s GMP regulations. GMP regulations require, among other things, quality control and quality assurance as well as the corresponding maintenance of records and documentation. The manufacturing facilities for our products must meet GMP requirements to the satisfaction of the FDA pursuant to a pre-approval inspection before Propanc can use them to manufacture products. Propanc and any third-party manufacturers are also subject to periodic inspections of facilities by the FDA and other authorities, including procedures and operations used in the testing and manufacture of our products to assess our compliance with applicable regulations.
With respect to post-market product advertising and promotion, the FDA imposes complex regulations on entities that advertise and promote pharmaceuticals, which include, among others, standards for direct-to-consumer advertising, promoting products for uses or in patient populations that are not described in the product’s approved labeling (known as “off-label use”), industry-sponsored scientific and educational activities and promotional activities involving the Internet. Failure to comply with FDA requirements can have negative consequences, including adverse publicity, enforcement letters from the FDA, mandated corrective advertising or communications with doctors and civil or criminal penalties. Although physicians may prescribe legally available drugs for off-label uses, manufacturers may not market or promote such off-label uses.
Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. A NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing a NDA.
Adverse event reporting and submission of periodic reports is required following FDA approval of a NDA. The FDA also may require post-marketing testing, known as Phase IV testing, risk mitigation strategies and surveillance to monitor the effects of an approved product or to place conditions on an approval that could restrict the distribution or use of the product.
Orphan Drug Designation
In June 2017, we were notified by the FDA that PRP had been granted orphan drug designation for the treatment of pancreatic cancer. Orphan drug designation may be granted by the FDA when a rare disease or condition is implicated and a potential treatment qualifies under the Orphan Drug Act and applicable FDA regulations. This qualifies us for various developmental incentives, including protocol assistance, the potential for research grants, the waiver of future application fees, and tax credits for clinical testing if we choose to host future clinical trials in the United States.
In October 2017, we submitted a request for a second orphan drug designation for PRP, this time for ovarian cancer.
On November 2, 2017, we were notified by the FDA that our request was not granted. The Office of Orphan Products Development (“OOPD”) stated that complete prevalence is used as a measure of disease in ovarian cancer, as this reflects the number of women who have been diagnosed with disease and may be eligible for treatment with the proposed therapy. Therefore, on the date of the submission of our application, the OOPD estimated that the prevalence of ovarian cancer was 228,110 cases. Since the prevalence exceeds the threshold of 200,000 to qualify for orphan drug designation, they could not grant our request. We may consider resubmitting our application if we can identify a suitable sub population in ovarian cancer, which may meet the target threshold.
| 55 |
European Union
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials, commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or market our product in those countries. The approval process varies from country to country and the time may differ than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country. Despite these differences, the clinical trials will be conducted according to international standards such as Good Clinical Practice (GCP), Good Manufacturing Practice (GMP) and Good Laboratory Practice (GLP), which is recognized by each foreign country under the International Conference of Harmonization (ICH) Guidelines. We will conduct our trials in each foreign jurisdiction according to these standards, undertaking a First-In-Human (FIH) Phase I study in patients with advanced solid tumors, evaluating the safety, pharmacokinetics, and anti-tumor efficacy of PRP. This will be followed by two Phase II studies evaluating the efficacy and safety of PRP. To ensure harmonization between the jurisdictions, we intend to conduct regulatory meetings in the country where trials are conducted, as well as the FDA and European Medicines Agency. A pre-IND (Investigational New Drug) meeting will be held with the FDA once initial patient data has been collected from the FIH study to ensure acceptability of future planned Phase II trials.
Under European Union regulatory systems, we must submit and obtain authorization for a clinical trial application in each member state in which we intend to conduct a clinical trial. After we have completed clinical trials, we must obtain marketing authorization before it can market its product. We must submit applications for marketing authorizations for oncology products under a centralized procedure. The centralized procedure provides for the grant of a single marketing authorization that is valid for all European Union member states. The European Medicines Agency (the “EMA”) is the agency responsible for the scientific evaluation of medicines that are to be assessed via the centralized procedure.
UK
On June 23, 2016, the UK government held a referendum to gauge voters’ support to remain or leave the European Union. The referendum resulted in 51.9% of UK voters in favor of leaving the European Union, commonly referred to as “Brexit.” On March 29, 2017, the UK invoked Article 50 of Lisbon Treaty to initiate complete withdrawal from the European Union by March 30, 2019. Currently, the center for the EMA is based in London but the European Union intends to relocate the center to another city.
The impact of Brexit on the drug approval process in the UK is uncertain, which could significantly impact Propanc as we may elect to conduct our clinical trials for PRP in the UK. Companies based in the UK and operating in the drug industry are urging the European Union and the UK to reach an agreement to harmonize the regulatory process once the UK officially exits the European Union. We hope to commence our Phase IIa trials in 2021, and we are hopeful that there will be greater clarity on the regulatory process for drug approvals in UK in the near future.
Australia
In Australia, the relevant regulatory body responsible for the pharmaceutical industry is the Therapeutics Goods Administration (the “TGA”). Prescription medicines are regulated under the Therapeutic Goods Act 1989. Under the Therapeutic Goods Act, the Therapeutic Goods Administration evaluates new products for quality, safety and efficacy before being approved for market authorization, according to similar standards employed by the FDA and EMA in the United States and European Union, respectively. However, receiving market authorization in one or two regions does not guarantee approval in another.
Third-Party Payor Coverage and Reimbursement
Although none of our product candidates have been commercialized for any indication, if they are approved for marketing, commercial success of our product candidates will depend, in part, upon the availability of coverage and reimbursement from third-party payors at the federal, state and private levels. In addition, in many countries outside the United States, a drug must be approved for reimbursement before it can be approved for sale in that country.
Eligibility for reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover costs and may not be made permanent. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies.
| 56 |
In many countries outside the United States, a drug must be approved for reimbursement before it can be approved for sale in that country. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval. We may not obtain foreign regulatory approvals on a timely basis, if at all. We may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our products in any foreign market.
Marketing Approvals, Pricing and Reimbursement Regulations
The regulations that govern marketing approvals, pricing and reimbursement for new drug products vary widely from country to country. In the United States, recently passed legislation may significantly change the approval requirements in ways that could involve additional costs and cause delays in obtaining approvals. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted.
Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products.
Other Regulations
We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances. We may incur significant costs to comply with such laws and regulations now or in the future.
Competition
The biotechnology and pharmaceutical industries are characterized by continuing technological advancement and significant competition. While we believe that our technology platforms, product candidates, know-how, experience and scientific resources provide us with competitive advantages, we face competition from major pharmaceutical and biotechnology companies, academic institutions, governmental agencies and public and private research institutions, among others. Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future. Key product features that would affect our ability to effectively compete with other therapeutics include the efficacy, safety and convenience of our products. The level of generic competition and the availability of reimbursement from government and other third-party payers will also significantly impact the pricing and competitiveness of our products. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market.
Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Our principal executive office is located at 302, 6 Butler Street, Camberwell, VIC, 3124 Australia, which we lease from Horizon Pty Ltd., a related party, of which Mr. Nathanielsz, our chief executive officer, chief financial officer and a director, and his wife are owners and directors. The lease has a five-year term commencing May 5, 2016, and we are obligated to pay $3,300 AUD or $2,325 USD (depending on exchange rate), inclusive of tax, in rent per month.
| 57 |
From time to time, we may be involved in litigation in the ordinary course of business. However, we are currently not involved in any litigation that we believe could have a material adverse effect on our financial condition or results of operations. To our knowledge, there is no action, suit, proceeding, inquiry or investigation before or by any court, public board, government agency, self-regulatory organization or body pending or, to the knowledge of our executive officers or any of our subsidiaries, threatened against or affecting our Company, our common stock, any of our subsidiaries or any of our subsidiaries’ officers or directors in their capacities as such, in which an adverse decision could have a material adverse effect.
MARKET PRICE OF AND DIVIDENDS ON OUR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information
Our common stock is quoted under the trading symbol “PPCB” on the OTC Markets - OTCQB. Only a limited market exists for our common stock. There is no assurance that a regular trading market will develop, or if developed, that it will be sustained. Therefore, a stockholder may be unable to resell his securities in our Company.
The following table sets forth the range of high and low bid quotations for our common stock for each of the periods indicated as reported by the OTCQB. These quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
| High
Bid* ($) | Low
Bid* ($) | |||||||
| Fiscal Year Ended June 30, 2019 | ||||||||
| Second quarter ended December 31, 2018 | $ | 0.0955 | 0.013 | |||||
| First quarter ended September 30, 2018 | $ | 0.2599 | 0.004 | |||||
| Fiscal Year Ended June 30, 2018 | ||||||||
| Fourth quarter ended June 30, 2018 | $ | 0.10 | 0.04 | |||||
| Third quarter ended March 31, 2018 | $ | 0.25 | 0.09 | |||||
| Second quarter ended December 31, 2017 | $ | 0.77 | 0.09 | |||||
| First quarter ended September 30, 2017 | $ | 1.10 | 0.23 | |||||
| Fiscal Year Ended June 30, 2017 | ||||||||
| Fourth quarter ended June 30, 2017 | $ | 2.70 | 0.90 | |||||
| Third quarter ended March 31, 2017 | $ | 3.83 | 2.03 | |||||
| Second quarter ended December 31, 2016 | $ | 4.25 | 1.68 | |||||
| First quarter ended September 30, 2016 | $ | 5.00 | 3.25 | |||||
* The quotations of the high and low prices reflect inter-dealer prices, without retail mark-up, markdown or commission.
On February 15, 2019, the last reported sales price per share of our common stock on the OTCQB was $0.015.
Number of Holders
As of February 14, 2019, we had 70 record holders of our common stock, 333,896,577 shares of common stock issued and outstanding, one holder of our Series A Preferred Stock holding 500,000 shares and one holder of our Series B Preferred Stock holding one share.
Penny Stock
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a market price of less than $5.00, other than securities registered on certain national securities exchanges or quoted on the NASDAQ system, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock, to deliver a standardized risk disclosure document prepared by the SEC, that: (a) contains a description of the nature and level of risk in the market for penny stocks in both public offerings and secondary trading; (b) contains a description of the broker’s or dealer’s duties to the customer and of the rights and remedies available to the customer with respect to a violation of such duties or other requirements of the securities laws; (c) contains a brief, clear, narrative description of a dealer market, including bid and ask prices for penny stocks and the significance of the spread between the bid and ask price; (d) contains a toll-free telephone number for inquiries on disciplinary actions; (e) defines significant terms in the disclosure document or in the conduct of trading in penny stocks; and (f) contains such other information and is in such form, including language, type size and format, as the SEC shall require by rule or regulation.
The broker-dealer also must provide, prior to effecting any transaction in a penny stock, the customer with (a) bid and offer quotations for the penny stock; (b) the compensation of the broker-dealer and its salesperson in the transaction; (c) the number of shares to which such bid and ask prices apply, or other comparable information relating to the depth and liquidity of the market for such stock; and (d) a monthly account statement showing the market value of each penny stock held in the customer’s account.
| 58 |
In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from those rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written acknowledgment of the receipt of a risk disclosure statement, a written agreement as to transactions involving penny stocks, and a signed and dated copy of a written suitability statement.
These disclosure requirements may have the effect of reducing the trading activity for our common stock. Therefore, stockholders may have difficulty selling our securities.
Dividends
We have not paid any cash dividends to our stockholders. The declaration of any future cash dividends is at the discretion of our Board and depends upon our earnings, if any, our capital requirements and financial position, and general economic conditions. It is our present intention not to pay any cash dividends in the foreseeable future, but rather to reinvest earnings, if any, in our business operations.
Securities Authorized for Issuance Under Equity Compensation Plans
| Plan Category | Number
of Securities to be Issued Upon Exercise of Outstanding Options | Weighted Average Exercise Price of Outstanding Options | Number
of Securities Remaining Available for Future Issuance Under Equity Compensation Plans | |||||||||
| Equity Compensation Plans Not Approved by Security Holders | 572,000 | (1) | $ | 7.50 | - | (2) | ||||||
| Total | 572,000 | (1) | $ | 7.50 | - | (2) | ||||||
| (1) | On April 14, 2016, our board of directors granted options to purchase shares of our common stock to each of James Nathanielsz, our Chief Executive Officer, Chief Financial Officer and a director, and Dr. Julian Kenyon, our director. We granted 286,000 stock options at an exercise price of $7.50 per share (market value of our shares on the grant date), to each of Mr. Nathanielsz and Mr. Kenyon. 95,333 of such options vested on April 14, 2016 and expire on April 14, 2021, 95,333 of such options vested on April 14, 2017 (first anniversary of the grant date) and expire on April 14, 2021, and 95,334 of such options vested on April 14, 2018 (second anniversary of the grant date) and expire on April 14, 2021. The fair value of each of the 286,000 options at the grant date is $1,962,440 (aggregate total of $3,924,880). | |
| (2) | Mr. Nathanielsz and Dr. Kenyon have the option under their individual employment and director agreements, respectively, to convert any accrued but unpaid salary or fees, as the case may be, into shares of our common stock at a conversion rate between par value and the closing bid price on the date of conversion to be determined by the parties. |
SELECTED FINANCIAL DATA
Not applicable to smaller reporting companies.
| 59 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and operating results together with our financial statements and related notes included elsewhere in this prospectus. This discussion and analysis and other parts of this prospectus contain forward-looking statements based upon current beliefs, plans and expectations that involve risks, uncertainties and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under “Risk Factors” or in other parts of this prospectus. The last day of our fiscal year is June 30. Our fiscal quarters end on September 30, December 31, March 31 and June 30 and our current fiscal year ended on June 30, 2018. U.S. Dollars are denoted herein by “USD,” “$” and “dollars”.
Overview
The Company was originally incorporated in Melbourne, Victoria Australia on October 15, 2007 as Propanc PTY LTD, and continues to be based in Camberwell, Victoria Australia. Since its inception, substantially all of the operations of the Company have been focused on the development of new cancer treatments targeting high-risk patients, particularly cancer survivors, who need a follow-up, non-toxic, long-term therapy designed to prevent the cancer from returning and spreading. The Company anticipates establishing global markets for its technologies. Our lead product candidate, which we refer to as PRP, is an enhanced pro-enzyme formulation designed to enhance the anti-cancer effects of multiple enzymes acting synergistically. PRP is currently in the preclinical phase of development.
On November 23, 2010, the Company was incorporated in the state of Delaware as Propanc Health Group Corporation. In January 2011, to reorganize the Company, we acquired all of the outstanding shares of Propanc PTY LTD on a one-for-one basis and Propanc PTY LTD became our wholly-owned subsidiary.
Effective April 20, 2017, the Company changed its name to “Propanc Biopharma, Inc.” to better reflect its current stage of operations and development.
To date, we have generated no revenue, have no cancer treatment products available to market and have no products which have reached the clinical trial stage. We require substantial additional financing to continue to test and commercialize PRP.
Recent Developments
Business Developments
BIO CEO & Investor Conference ―In February 2019, we presented at the 2019 BIO CEO & Investor Conference held at the New York Marriott Marquis. This conference is one of the largest investor conferences focused on established and emerging publicly traded and select private biotech companies. As part of our presentation and one-on-one meetings, we provided a Company update for 2019, including our focus on the advancement of our lead product, PRP, in 2019.
Partnership with University of Jaén― In January 2019, we announced that a cooperation agreement has been entered into between the University of Jaén and our Company to commence the POP1 joint drug discovery program to be co-funded by both parties. The agreement coincides with the appointment of research scientist, Mr. Aitor González, to lead the drug discovery and research activities over the next 3 to 4 years. The objective of the program is to identify and develop suitable backup compounds to our lead product candidate, PRP.As part of the agreement, Macarena Perán, Ph.D. and Julian Kenyon, M.D. have been appointed as joint supervisors, representing the University and our Company, respectively. The program involves advancing new compounds through a drug screening process, followed by preclinical and early stage clinical development. As the drug candidate progresses along the development pathway, the collaboration will also involve the Universities of Granada and Jaén, as well as Granada and Almeria Hospitals, which are members of FIBAO, a Public Health Foundation, based in Granada, Spain, committed to assisting commercial partners with the development and commercialization of innovative technologies designed to benefit humankind.
Approval of Foundation Patent in India ― In December 2018, we announced that our foundation patent application has been granted by the Office of the Controller General of Patents, Design and Trademarks, India. The foundation patent, which covers our lead product candidate, PRP, pioneers the discovery of a pharmaceutical composition for treating cancer via a combination of trypsinogen and/or chymotrypsinogen pancreatic proenzymes. As of December 31, 2018, the foundation patent has been granted in the USA, Europe (including Belgium, Czech Republic, Denmark, France, Germany, Ireland, Italy, the Netherlands, Portugal, Spain, Sweden, Switzerland/Liechtenstein, Turkey and the United Kingdom), China, Japan, Indonesia, Malaysia, Israel, Australia, New Zealand, Singapore, South Africa, Mexico, Republic of Korea, Hong Kong and more recently, India. It is presently under examination in Canada and Brazil.
LaVoieHealthScience ― In December 2018, we appointed LaVoieHealthScience (“LHS”) as our communications agency of record. LHS is an integrated investor and public relations agency focused on advancing health and science innovations. We are partnering with LHS to develop corporate awareness of our Company as a health science innovator as we plan to transition our lead technology into human clinical trials in 2019.
| 60 |
Financing Transactions
Equity Line ― On February 25, 2019, we entered into the Equity Purchase Agreement with Oasis Capital (the “Oasis Equity Purchase Agreement”) pursuant to which Oasis Capital committed to purchase up to $10,000,000 shares of our common stock (the “Oasis Equity Line”).
On February 4, 2019, we entered into a letter agreement with a certain investment bank, pursuant to which we retained such investment bank as our exclusive placement agent through May 31, 2019. As of the date of this prospectus, we have not entered into any financing arrangements or received any funding in connection with such agreement or the efforts of such placement agent.
Eagle Equities Convertible Note ―On December 24, 2018, we entered into a securities purchase agreement with Eagle Equities, LLC (“Eagle Equities”), pursuant to which Eagle Equities purchased an 8% convertible promissory note (the “December 2018 Eagle Note”) from us in the principal amount of $126,000, convertible into shares of our common stock at the option of Eagle Equities at any time after the six-month anniversary of the December 2018 Eagle Note. The maturity date of the December 2018 Eagle Note is December 24, 2019.
Eagle Equities Convertible Note ― On November 30, 2018, we entered into a securities purchase agreement with Eagle Equities, pursuant to which Eagle Equities purchased an 8% convertible promissory note (the “November 2018 Eagle Note”) from us in the principal amount of $105,000, convertible into shares of our common stock at the option of Eagle Equities at any time after the six-month anniversary of the November 2018 Eagle Note. The maturity date of the November 2018 Eagle Note is November 30, 2019.
Equity Line ― On October 5, 2018, we entered into the Equity Purchase Agreement (the “L2 Equity Purchase Agreement”) with L2 Capital, LLC (“L2 Capital”) pursuant to which L2 Capital committed to purchase up to $10,000,000 shares of our common stock (the “L2 Equity Line”). In connection with the execution of the L2 Equity Purchase Agreement, we issued 3,850,597 shares of our common stock to L2 Capital as a commitment fee, which are subject to a lock-up/leak-out limitation as described elsewhere in this registration statement of which this prospectus is a part. We do not intend to utilize the L2 Equity Line in the future.
Eagle Equities Convertible Note ― Effective October 2, 2018, the Company entered into a securities purchase agreement with Eagle Equities, pursuant to which Eagle Equities purchased a convertible promissory note (the “October 2018 Eagle Note”) from the Company in the aggregate principal amount of $210,000.00, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Eagle Equities any time after the six-month anniversary of the October 2018 Eagle Note. The transactions contemplated by the securities purchase agreement closed on October 3, 2018. Pursuant to the terms of the securities purchase agreement, Eagle Equities deducted $10,000.00 from the principal payment due under the October 2018 Eagle Note, at the time of closing, to be applied to its legal expenses. The maturity date of the October 2018 Eagle Note is October 2, 2019. The October 2018 Eagle Note bears interest at a rate of 8% per annum, which interest shall be paid by the Company in shares of its common stock upon receipt of a notice of conversion by the Company from Eagle Equities at any time after the six month anniversary of the October 2018 Eagle Note. Eagle Equities has the option to convert all or any amount of the principal face amount of the October 2018 Eagle Note, at any time, for shares of the Company’s common stock at a price equal to 60% of the lowest closing bid price of the Company’s common stock for the ten prior trading days, including the day upon which the Company receives a notice of conversion from Eagle Equities. Eagle Equities is restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Eagle Equities and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock. The October 2018 Eagle Note may be prepaid by the Company on the terms set forth in the note.
Coventry Enterprises Convertible Note ― Effective October 2, 2018, the Company entered into a securities purchase agreement with GS Capital Partners, LLC (“GS Capital”), pursuant to which GS Capital purchased two 8% unsecured convertible promissory notes from the Company in the aggregate principal amount of $212,000.00, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of GS Capital. The purchase price of $106,000 of the first note (the “GS Capital First Note”) was paid in cash by GS Capital on October 3, 2018. After payment of certain legal fees and expenses, net proceeds to the Company from the GS Capital First Note totaled $100,700. The purchase price of $106,000 of the second note (the “GS Capital Back End Note”) was initially paid for by the issuance of an offsetting $106,000 collateralized secured note issued to Company by GS Capital (the “GS Capital Enterprises Note”). The terms and conditions of the GS Capital Back End Note are substantially the same as the GS Capital First Note, with the following exceptions: the GS Capital Back End Note is not convertible until it is funded in cash on or before June 2, 2019; provided that in no event will GS Capital be entitled to fund the GS Capital Back End Note in cash if (i) the Company’s common stock has a closing bid price of less than $0.045 per share for at least five consecutive trading days immediately prior to such funding, or (ii) the aggregate dollar trading volume of the Company’s common stock is less $40,000 in any five consecutive trading days immediately prior to such funding. The maturity date of the GS Capital First Note is October 2, 2019. The GS Capital First Note bears interest at a rate of 8% per annum, which interest shall be paid by the Company in shares of the Company’s common stock at any time GS Capital sends a notice of conversion to the Company. GS Capital is entitled to, at its option, convert all or any amount of the principal face amount and any accrued but unpaid interest of the GS Capital First Note into shares of the Company’s common stock, at any time after April 2, 2019, at a conversion price for each share of common stock equal to 61% of the lowest closing bid price of the Company’s common stock for the ten prior trading days including the day upon which a notice of conversion is received by the Company from GS Capital. GS Capital is restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by GS Capital and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock. The GS Capital First Note may be prepaid by the Company on the terms set forth in the note.
| 61 |
Critical Accounting Estimates
Below is a discussion of our more subjective accounting estimation processes for purposes of explaining (i) the methodology used in calculating the estimates, (ii) the inherent uncertainties pertaining to such estimates and (iii) the possible effects of a significant variance in actual experience, from that of the estimate, on the Company’s financial condition. Estimates involve numerous assumptions that, if incorrect, could create a material adverse impact on the Company’s results of operations and financial condition.
Reference is frequently made herein to the Financial Accounting Standards Board (the “FASB”) Accounting Standards Codification (“ASC”). This is the source of authoritative US GAAP recognized by the FASB to be applied to non-governmental entities. Each ASC reference in this filing is presented with a three-digit number, which represents its Topic. As necessary for explanation and as applicable, an ASC topic may be followed with a two-digit subtopic, a two-digit section or a two-or-three digit paragraph.
Foreign Currency Translation and Comprehensive Income (Loss): The Company’s functional currency is the Australian Dollar (“AUD”). For financial reporting purposes, the AUD has been translated into USD as the reporting currency. Assets and liabilities are translated at the exchange rate in effect at the balance sheet date. Revenues and expenses are translated at the average rate of exchange prevailing during the reporting period. Equity transactions are translated at each historical transaction date spot rate. Translation adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’ equity (deficit) as “accumulated other comprehensive income (loss).” Gains and losses resulting from foreign currency transactions are included in the statement of operations and comprehensive loss as other income (expense).
Accounting for Income Taxes: The Company is governed by Australia and United States income tax laws, which are administered by the Australian Taxation Office and the United States Internal Revenue Service, respectively. The Company follows ASC 740, “Accounting for Income Taxes,” which requires an asset and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed annually for temporary differences between the financial statements and tax bases of assets and liabilities that will result in taxable or deductible amounts in the future based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Income tax expense is the tax payable or refundable for the period plus or minus the change during the period in deferred tax assets and liabilities.
The Company adopted provisions of ASC 740, Sections 25 through 60, “Accounting for Uncertainty in Income Taxes.” These sections provide detailed guidance for the financial statement recognition, measurement and disclosure of uncertain tax positions recognized in the financial statements. Tax positions must meet a “more-likely-than-not” recognition threshold at the effective date to be recognized upon the adoption of ASC 740 and in subsequent periods.
Accounting for Stock Based Compensation: The Company records stock based compensation in accordance with ASC 718, “Stock Compensation” and Staff Accounting Bulletin No. 107 issued by the SEC in March 2005 regarding its interpretation of ASC 718. ASC 718 requires the fair value of all stock-based employee compensation awarded to employees to be recorded as an expense over the related requisite service period. The statement also requires the recognition of compensation expense for the fair value of any unvested stock option awards outstanding at the date of adoption. The Company values any employee or non-employee stock based compensation at fair value using the Black-Scholes Option Pricing Model.
The Company accounts for non-employee share-based awards in accordance with the measurement and recognition criteria of ASC 505-50 “Equity-Based Payments to Non-Employees.”
Derivative Instruments: ASC 815, “Derivatives and Hedging,” establishes accounting and reporting standards for derivative instruments and for hedging activities by requiring that all derivatives be recognized in the balance sheet and measured at fair value. Gains or losses resulting from changes in the fair value of derivatives are recognized in earnings. On the date of conversion or payoff of debt, the company records the fair value of the conversion shares, removes the fair value of the related derivative liability, removes any discounts and records a net gain or loss on debt extinguishment.
Convertible Notes With Variable Conversion Options: The Company has entered into convertible notes, some of which contain variable conversion options, whereby the outstanding principal and accrued interest may be converted, by the holder, into common shares at a fixed discount to the price of the common stock at the time of conversion. The Company treats these convertible notes as stock settled debt under ASC 480 and measures the fair value of the notes at the time of issuance, which is the result of the share price discount at the time of conversion, and records the put premium as accretion to interest expense to the date of first conversion.
| 62 |
Research and Development Tax Credits: The Company may apply for research and development tax concessions with the Australian Taxation Office on an annual basis. Although the amount is possible to estimate at year end, the Australian Taxation Office may reject or materially alter the claim amount. Accordingly, the Company does not recognize the benefit of the claim amount until cash receipt since collectability is not certain until such time. The tax concession is a refundable credit. If the Company has net income then the Company can receive the credit which reduces its income tax liability. If the Company has net losses, then the Company may still receive a cash payment for the credit, however, the Company’s net operating loss carry forwards are reduced by the gross equivalent loss that would produce the credit amount when the income tax rate is applied to that gross amount. The concession is recognized as an income tax benefit, in operations, upon receipt.
Recent Accounting Pronouncements
Certain FASB Accounting Standard Updates (“ASU”) which are not effective until after June 30, 2018 are not expected to have a significant effect on the Company’s consolidated financial position or results of operations. The Company is evaluating or has implemented the following at June 30, 2018:
ASU 2018-07 - In June 2018, the Financial Accounting Standards Board (the “FASB”) issued Accounting Standards Update (“ASU”) 2018-07, Compensation – Stock Compensation (Topic 718). This update is intended to reduce cost and complexity and to improve financial reporting for share-based payments issued to non-employees (for example, service providers, external legal counsel, suppliers, etc.). The ASU expands the scope of Topic 718, Compensation—Stock Compensation, which currently only includes share-based payments issued to employees, to also include share-based payments issued to non-employees for goods and services. Consequently, the accounting for share-based payments to non-employees and employees will be substantially aligned. This standard will be effective for financial statements issued by public companies for the annual and interim periods beginning after December 15, 2018. Early adoption of the standard is permitted. The standard will be applied in a retrospective approach for each period presented. Management currently does not plan to early adopt this guidance and is evaluating the potential impact of this guidance on the Company’s consolidated financial statements as well as transition methods.
ASU 2017-01 - In January 2017, the FASB issued ASU No. 2017-01: “Business Combinations (Topic 805)- to clarify the definition of a business with the objective of adding guidance to assist entities with evaluating whether transactions should be accounted for as acquisitions (or disposals) of assets or businesses. This guidance is effective for interim and annual reporting periods beginning after December 15, 2017. The Company implemented this guidance effective January 1, 2018.
ASU No 2016-18 – In November 2016, the FASB issue ASU No. 2016-18, Statement of Cash Flows (Topic 230) Restricted Cash (ASU 2016-18), requiring restricted cash and cash equivalents to be included with cash and cash equivalents of the statement of cash flows. The new standard is effective for fiscal years, and interim periods with those year, beginning December 15, 2017, with early adoption permitted. The Company has elected to adopt this new ASU at July 1, 2018 and does not anticipate the ASU to have a material impact on its consolidated financial statements.
ASU 2016-02 - In February 2016, the FASB issued ASU No. 2016-02: “Leases (Topic 842)” whereby lessees will need to recognize almost all leases on their balance sheet as a right of use asset and a lease liability. This guidance is effective for interim and annual reporting periods beginning after December 15, 2018. The Company does not anticipate the ASU to have a material impact on its consolidated financial statements.
ASU 2014-09 - In May 2014, the FASB issued ASU No. 2014-09: “Revenue from Contracts with Customers (Topic 606)” which requires that an entity recognize revenue to depict the transfer of promised goods and services to customers in an amount that reflects the consideration to which the Company expects to be entitled in exchange for those goods or services. Since the issuance of the original standard, the FASB has issued several updates to the standard which (i) clarify the application of the principal versus agent guidance; (ii) clarify the guidance relating to performance obligations and licensing; (iii) clarify assessment of the collectability criterion, presentation of sales taxes, measurement date for non-cash consideration and completed contracts at transaction; and (iv) clarify narrow aspects of ASC 606 or corrects unintended application of the guidance. The new revenue recognition standard, amended by the updates, becomes effective in the first quarter of fiscal 2019 and is to be applied retrospectively using one of two prescribed methods. Early adoption is permitted. The Company adopted the new standard effective July 1, 2018.
| 63 |
Results of Operations
The following discussion of our results of operations for the periods presented should be read in conjunction with our consolidated financial statements and notes thereto included elsewhere in this prospectus. The results discussed below are of our Company and our wholly-owned Australian subsidiary, Propanc PTY LTD.
For the Three Months Ended December 31, 2018, as compared to the Three Months Ended December 31, 2017
Revenue
For the three months ended December 31, 2018 and 2017 we generated no revenue because we are currently undertaking research and development activities for market approval and no sales were generated in this period.
Administration Expense
Administration expense increased by approximately $261,000 to $656,594 for the three months ended December 31, 2018, as compared to $395,442 for the three months ended December 31, 2017. This increase is primarily attributable to an increase of approximately $97,600 in investor relations expense, an increase of approximately $97,900 in legal fees and an increase of approximately $167,000 in annual leave expense for the three months ended December 31, 2018, offset by a decrease in stock based expense of approximately $107,900 that was related to a grant of stock options to our directors in April 2016.
Occupancy Expense
Occupancy expense decreased by approximately $450 to $6,449 for the three months ended December 31, 2018, as compared to $6,899 for the three months ended December 31, 2017. The decrease primarily relates to the fluctuation in foreign currency exchange rates of approximately $450 in the three months ended December 31, 2018.
Research and Development Expenses
Research and development expenses decreased by approximately $939,952 to $94,777 for the three months ended December 31, 2018, as compared to $1,034,729 for the three months ended December 31, 2017. The decrease in research and development expenditures is primarily attributable to completion of process development activities and preparation for commencement of the engineering run and subsequent GMP manufacture of PRP for clinical trials with the process, preparation and manufacture having been completed in the period ended December 31, 2017, which clinical trials we hope to commence if we raise sufficient proceeds in 2019 calendar year via the Equity Line or by raising additional capital. This includes raw material purification and stabilization process development, development of analytical quality assurance and control methods, reproduction runs for raw materials, and preparation of raw materials and finished product specifications for future full scale GMP manufacture of PRP.
Interest Expense/Income
Interest expense increased by approximately $8,200 to $494,989 for the three months ended December 31, 2018, as compared to $486,754 for the three months ended December 31, 2017. Interest expense is primarily comprised of approximately $85,900 of debt discount amortization and approximately $355,500 accretion of debt premium. This increase is primarily attributable to a decrease in the issuance of derivative debt resulting in lower amortization of debt discount offset by higher accretion amounts of convertible notes with discounted debt features during the quarter ended December 31, 2018.
Change in Fair Value of Derivative Liabilities
Change in fair value of derivative liabilities increased by approximately $299,500 to a gain of $39,470 for the three months ended December 31, 2018, as compared to a loss of $260,045 for the three months ended December 31, 2017. This increase is primarily attributable to the decrease in derivative debt on our books due to full conversion or payoff of convertible notes during the three months ended December 31, 2018.
Gain (loss) on Debt Settlements, Net
Gain (loss) on settlement of debt decreased by approximately $28,000 to a loss of $132 for the three months ended December 31, 2018, as compared with a gain of $28,244 for the three months ended December 31, 2017. The decrease in gain (loss) on settlement of debt is primarily attributable to the write off of old accruals for the three months ended December 31, 2017.
| 64 |
Foreign Currency Transaction Loss
Foreign currency transaction increased by approximately $190,000 to a loss of $333,205 for the three months ended December 31, 2018, as compared with a loss of $142,944 for the three months ended December 31, 2017. The increase in foreign currency transaction loss is primarily attributable to greater fluctuation in exchange rates in the three months ended December 31, 2018, as compared to the three months ended December 31, 2017.
Net loss
Net loss decreased by approximately $751,900 to $1,450,038 for the three months ended December 31, 2018, as compared to a net loss of $2,201,979 for the three months ended December 31, 2017. The decrease is primarily attributable a decrease in operating loss of approximately $679,000 and a decrease in the gain on debt extinguishment of approximately $63,000.
For the Six Months Ended December 31, 2018, as compared to the Six Months Ended December 31, 2017
Revenue
For the six months ended December 31, 2018 and 2017 we generated no revenue because we are currently undertaking research and development activities for market approval and no sales were generated in this period.
Administration Expense
Administration expense increased by approximately $14,300 to $1,041,194 for the six months ended December 31, 2018, as compared to $1,026,848 for the six months ended December 31, 2017. This increase is primarily attributable to an increase of approximately $154,000 in investor relations expense, an increase of approximately $99,000 in legal fees and an increase of approximately $177,000 in employee leave for the six months ended December 31, 2018, offset by a decrease in stock based expense of approximately $262,000 that was related to a grant of stock options to our directors in April 2016, a decrease in consulting expense of approximately $146,000.
Occupancy Expense
Occupancy expense decreased by approximately $1,200 to $14,527 for the six months ended December 31, 2018, as compared to $15,729 for the six months ended December 31, 2017. The decrease primarily relates to the fluctuation in foreign currency exchange rates of approximately $1,100 in the six months ended December 31, 2018.
Research and Development Expenses
Research and development expenses decreased by approximately $1,477,500 to $150,970 for the six months ended December 31, 2018, as compared to $1,598,468 for the six months ended December 31, 2017. The decrease in research and development expenditures is primarily attributable to completion of process development activities and preparation for commencement of the engineering run and subsequent GMP manufacture of PRP for clinical trials in the six months ended December 31, 2017, which clinical trials we hope to commence if we raise sufficient proceeds in 2019 calendar year via the Equity Line or by raising additional capital. This includes raw material purification and stabilization process development, development of analytical quality assurance and control methods, reproduction runs for raw materials, and preparation of raw materials and finished product specifications for future full scale GMP manufacture of PRP.
Interest Expense/Income
Interest expense decreased by approximately $259,700 to $1,119,527 for the six months ended December 31, 2018, as compared to $1,379,186 for the six months ended December 31, 2017. Interest expense is primarily comprised of approximately $304,000 of debt discount amortization and approximately $699,000 of accretion of debt premium. This decrease is primarily attributable to a decrease in the issuance of derivative debt resulting in lower amortization of debt discount, along with a decrease in issuance of convertible notes with discounted debt features during the quarter ended December 31, 2018.
Change in Fair Value of Derivative Liabilities
Change in fair value of derivative liabilities increased by approximately $1,702,000 to a loss of $1,931,646 for the six months ended December 31, 2018, as compared to a loss of $229,771 for the six months ended December 31, 2017. This increase is primarily attributable to an increase in the volatility of the prices of our shares of common stock along with a decrease in stock price during the quarter ended December 31, 2018, which resulted in the recognition of such greater loss.
| 65 |
Gain (loss) on Debt Settlements, Net
Gain (loss) on settlement of debt decreased by approximately $22,500 to a gain of $14,289 for the six months ended December 31, 2018, as compared with a gain of $36,814 for the six months ended December 31, 2017. The decrease in gain (loss) on settlement of debt is primarily attributable to fewer debt settlement agreements entered into in the six months ended December 31, 2018.
Foreign Currency Transaction Gain (Loss)
Foreign currency transaction increased by approximately $604,800 to a loss of $613,912 for the six months ended December 31, 2018, as compared with a loss of $9,152 for the six months ended December 31, 2017. The increase in foreign currency transaction loss is primarily attributable to greater fluctuation in exchange rates in the six months ended December 31, 2018, as compared to the six months ended December 31, 2017.
Net loss
Net loss decreased by approximately $551,000 to $3,574,974 for the six months ended December 31, 2018, as compared to a net loss of $4,125,722 for the six months ended December 31, 2017. The decrease is primarily attributable to an increase in unrealized loss resulting in change in fair value of derivative liabilities of approximately $1,702,000, an increase in the gain of debt extinguishment of approximately $1,249,000 offset by a decrease in operating loss of approximately $1,434,000.
Fiscal Year Ended June 30, 2018, as compared to the Fiscal Year Ended June 30, 2017
Revenue
For the fiscal years 2018 and 2017 we generated no revenue because we are currently undertaking research and development activities for market approval and no sales were generated in this period.
Administration Expense
Administration expense decreased to $2,103,684 for the year ended June 30, 2018 as compared to $4,739,431 for the year ended June 30, 2017. This decrease is primarily attributable to a decrease in stock based expense of approximately $1,170,000 that was related to a grant of stock options to our directors in April 2016 along with a decrease of approximately $1,020,000 that is primarily related to a decrease in stock based consulting fees, a decrease of approximately $230,000 in investor relations expense and a decrease in legal expenses of approximately $165,000 in the year ended June 30, 2018.
Occupancy Expense
Occupancy expense increased by approximately $1,500 to $30,521 for the year ended June 30, 2018. The increase relates to the fluctuation in foreign currency exchange rates along with an approximately $700 decrease in occupancy expense in the year ended June 30, 2018 related to a reclassification of expenses that occurred in the prior year.
Research and Development Expenses
Research and development expenses were $1,825,728 for the year ended June 30, 2018, as compared to $971,769 for the year ended June 30, 2017. The increase in research and development expenditures is primarily attributable to an increase in manufacturing and process development activities as the Company progresses its lead product, PRP, to clinical trials. This includes raw material purification and stabilization process development, development of analytical quality assurance and control methods, reproduction runs for raw materials, and preparation of raw materials and finished product specifications for future full scale GMP manufacture of PRP.
Interest Expense/Income
Interest expense decreased to $2,789,196 for the year ended June 30, 2018, as compared to $3,202,774 for the year ended June 30, 2017. Interest expense is primarily comprised of approximately $853,000 of debt discount amortization, and approximately $1,784,000 accretion of debt premium. This decrease is primarily attributable to a decrease in the issuance of derivative debt resulting in lower amortization of debt discount offset by higher accretion amounts of convertible notes with discounted debt features during the year ended June 30, 2018.
Change in Fair Value of Derivative Liabilities
Change in fair value of derivative liabilities decreased by $827,765 to a loss of $(7,612) for the year ended June 30, 2018, as compared to a gain of $820,153 for the year ended June 30, 2017. This decrease is primarily attributable to an increase in the volatility of the Company’s stock along with a decrease in stock price during the year ended June 30, 2018, which resulted in the recognition of such loss.
| 66 |
Loss on Debt Settlements, Net
Loss on settlement of debt decreased by $177,065 to a loss of $(18,585) for the year ended June 30, 2018, as compared with a loss of $(195,650) for the year ended June 30, 2017. The decrease in loss on debt settlements is primarily attributable to fewer fair market value price adjustments in the year ended June 30, 2018 along with approximately $36,000 in write-offs of old accruals.
Foreign Currency Transaction Gain (Loss)
Foreign currency transaction decreased to a loss of $(694,614) for the year ended June 30, 2018 as compared with a gain of $144,605 for the year ended June 30, 2017. The decrease in foreign currency transaction gain is primarily attributable to greater fluctuation in exchange rates in the year ended June 30, 2018 as compared to the year ended June 30, 2017.
Income Tax Benefit
During the years ended June 30, 2018 and 2017, we applied for and received from the Australian Taxation Office a research and development tax credit in the amount of $179,306 and $305,673, respectively.
Net loss
Net loss decreased to $(7,039,155) for the year ended June 30, 2018 as compared to a net loss of $(7,867,500) for the year ended June 30, 2017. The decrease is primarily attributable to a decrease in operating loss of approximately $1,780,000 and fluctuations in unrealized gains and losses in the year ended June 30, 2018.
Liquidity and Capital Resources
Current Financial Condition
As of December 31, 2018, we had total assets of $712,597, comprised primarily of cash of $124,538, GST tax receivable of $8,639, prepaid expenses and other current assets of $254,878, deferred offering costs of $313,923 and property and equipment, net, of $8,505. This compares with total assets of $71,387 as of June 30, 2018, comprised primarily of cash of $19,921, GST tax receivable of $6,257, prepaid expenses and other current assets of $34,712 and property and equipment, net, of $8,277.
As of December 31, 2018, we had current liabilities of $4,616,633, primarily comprised of net convertible debt of $2,798,624, accounts payable and accrued expenses of $1,359,531, employee benefit liability of $308,519 and embedded conversion option liabilities of $66,490. This compares with current liabilities of $6,823,307, primarily comprised of net convertible debt of $4,699,299, accounts payable and accrued expenses of $1,521,773 and embedded conversion option liabilities of $371,532, as of June 30, 2018.
We have funded and continue to fund our operations primarily through the issuance of equity and/or convertible securities for cash. The cash was used primarily for payments for research and development, administration expenses, occupancy expenses, professional fees, consultants’ expenses and travel expenses.
During the six months ended December 31, 2018 and as of the date of this Quarterly Report, we borrowed gross proceeds of approximately $1,100,000 from the sale of convertible promissory notes during with various maturity dates ranging from June 29, 2019 to December 24, 2019. In addition, during the six months ended December 31, 2018 and as of February 14, 2019, we received gross proceeds of approximately $1,000,000 from the put notices that we submitted to L2 Capital as of such date, to purchase an aggregate of 56,600,000 shares of our common stock. We do not intend to utilize the L2 Equity Line in the future. As of about February 7, 2019, we reached the maximum number of shares that we can put under the L2 Equity Line, and therefore, we would have had to file with the SEC a new registration statement registering additional shares under the L2 Equity Line if we had determined to continue to utilize the L2 Equity Line. Effective as of February 25, 2019, we terminated the L2 Equity Line.
| 67 |
We have substantial capital resource requirements and have incurred significant losses since inception. As of December 31, 2018, we had $124,538 in cash. We depend upon debt and/or equity financing to fund our ongoing operations and to execute our current business plan. Such capital requirements are in excess of what we have in available cash and for which we currently have commitments. Therefore, we presently do not have enough available cash to meet our obligations over the next 12 months. We intend to use the proceeds of the Oasis Equity Line, subject to the effectiveness of this registration statement of which this prospectus is a part, or seek other financing to generate additional cash to fund our operations and business expenses, including research and development. If we are unable to access the Oasis Equity Line, we believe our current available cash may be insufficient to meet our cash needs for the near future, including for planned clinical trials. We may then need to borrow additional amounts via the issuance of convertible notes on terms similar to the notes described in this prospectus and/or obtain alternative or additional financing from financial institutions, investors or otherwise, in order to maintain and expand our existing operations. In addition, on February 4, 2019, we entered into a letter agreement with a certain investment bank, pursuant to which we retained such investment bank as our exclusive placement agent through May 31, 2019. As of the date of this prospectus, we have not entered into any financing arrangements or received any funding in connection with such agreement or the efforts of such placement agent. In the event of the closing of an offering during such period (or the tail period after, if applicable), such investment bank would receive a percentage of the proceeds in cash and a percentage of the shares of our common stock issued in the offering as warrants. There can be no assurances that the Oasis Equity Line or any other financing will be available in amounts or terms acceptable to us, if at all. The failure by us to obtain such financing at reasonable terms would have a material adverse effect upon our business and plan of operations, financial condition and results of operations, and adversely affect our ability to complete ongoing activities in connection with our research and development programs, including initiating planned clinical trials.
Sources and Uses of Cash for the Six Months Ended December 31, 2018
| For
the Six Months Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
| Net cash used in operating activities | $ | (1,056,786 | ) | $ | (1,071,326 | ) | ||
| Net cash used in investing activities | $ | (1,662 | ) | $ | - | |||
| Net cash provided by financing activities | $ | 1,225,892 | $ | 1,274,000 | ||||
| Effect of exchange rate changes on cash | $ | (62,827 | ) | $ | (159,221 | ) | ||
Net cash used in operating activities was $1,056,786 for the six months ended December 31, 2018, as compared to $1,071,326 for the six months ended December 31, 2017. This fluctuation is due to a decrease in accounts payable of approximately $980,000, along with fluctuations in changes in foreign currency transaction gains and losses, changes related to the valuation of new derivative liabilities and the revaluation of existing derivative liabilities in the six months ended December 31, 2018.
Net cash used in investing activities was $1,662 for the six months ended December 31, 2018, as compared to $0 for the six months ended December 31, 2017. This fluctuation is due to the purchase of fixed assets in the six months ended December 31, 2018.
Cash flows provided by financing activities for the six months ended December 31, 2018 were $1,225,892, as compared to $1,274,000 for the six months ended December 31, 2017. During the six months ended December 31, 2018, we received proceeds from the sale of convertible promissory notes of $1,054,089. During the six months ended December 31, 2017, we received proceeds from convertible promissory notes of $1,274,000.
The effect of the exchange rate on cash resulted in a $62,827 negative adjustment to cash flows in the six months ended December 31, 2018, as compared to a negative adjustment of $159,211 to cash flows in the six months ended December 31, 2017. The reason for the fluctuation is due to the application of currency translation rates throughout the cash flow statement, the volume of transactions within each period and the daily fluctuation in exchange rates.
Sources and Uses of Cash for the Fiscal Year Ended June 30, 2018
| For
the Fiscal Year Ended June 30, | ||||||||
| 2018 | 2017 | |||||||
| Net cash used in operating activities | $ | (2,177,645 | ) | $ | (2,050,636 | ) | ||
| Net cash used in investing activities | $ | - | $ | - | ||||
| Net cash provided by financing activities | $ | 2,396,488 | $ | 2,098,786 | ||||
| Effect of exchange rate changes on cash | $ | (267,965 | ) | $ | (100,177 | ) | ||
Net cash used in operating activities was $2,177,645 for the fiscal year ended June 30, 2018 compared to $2,050,636 for the fiscal year ended June 30, 2017. This fluctuation is due to a decrease in stock option expense of approximately $1,100,000, offset by an increase in accounts payable of approximately $600,000 primarily related to research and development expenses, along with fluctuations in changes in foreign currency transaction gains and losses, changes related to the valuation of new derivative liabilities and the revaluation of existing derivative liabilities in the year ended June 30, 2018.
Cash flows provided by financing activities for the fiscal year ended June 30, 2018 were $2,396,488 as compared to $2,098,786 for the fiscal year ended June 30, 2017. During the year ended June 30, 2018, we received proceeds from the sale of convertible promissory notes of $2,890,080. During the year ended June 30, 2017, we received proceeds from convertible promissory notes of $1,634,500 and proceeds from the exercise of warrants of $464,286.
| 68 |
The effect of the exchange rate on cash resulted in a $267,965 negative adjustment to cash flows in the year ended June 30, 2018 as compared to a negative adjustment of $100,177 to cash flows in the year ended June 30, 2017. The reason for the fluctuation is due to the application of currency translation rates throughout the cash flow statement, the volume of transactions within each period and the daily fluctuation in exchange rates.
Going Concern Qualification
We did not generate any revenue for the six months ended December 31, 2018 and 2017 and have incurred significant losses and cash used in operations, and such losses and use of cash are expected to continue. Our independent registered public accounting firm has included a “Going Concern Qualification” in their audit report for each of the fiscal years ended June 30, 2018 and 2017. In addition, we have negative working capital and convertible debt that is past maturity that we are currently negotiating with lenders in order to amend the maturity dates. The foregoing raises substantial doubt about our ability to continue as a going concern. Our ability to continue as a going concern is dependent on our ability to execute our strategy and on our ability to raise additional funds. Management is currently seeking additional funds to operate our business, primarily through the issuance of equity and/or debt securities for cash, including continuing to utilize the Equity Line. No assurance can be given that the Equity Line will continue to be available or other financing will be available or, if available, that it will be in amounts or on terms that are satisfactory to us. Even if we are able to obtain additional financing, it may contain undue restrictions on our operations, in the case of debt financing or cause substantial dilution for our stockholders, in case of equity and/or convertible debt financing. The condensed consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. The “Going Concern Qualification” might make it substantially more difficult to raise capital.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources.
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not applicable to smaller reporting companies.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
There have been no changes in our independent registered public accounting firm during the last two fiscal years, and we have not had any material disagreements with our independent registered public accounting firm during that time.
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The following table sets forth certain information regarding our current executive officers and directors as of February 14, 2019:
| Name | Age | Position | ||
| James Nathanielsz | 44 | Chief Executive Officer, Chief Financial Officer, Acting Chairman, Secretary, Treasurer and Director | ||
| Dr. Julian Kenyon | 71 | Director |
Executive Officers and Directors
James Nathanielsz― Mr. Nathanielsz has served as a director of our Company since inception. Mr. Nathanielsz has served as a director and chief executive officer of our Australian subsidiary since October 2007. From July 2006 until October 2007, Mr. Nathanielsz served as the New Products Manager of Biota Holdings Limited, an anti-infective drug development company in Australia. Mr. Nathanielsz graduated with a Bachelor of Applied Science, majoring in Biochemistry/Applied Chemistry and with a Master of Entrepreneurship & Innovation from Swinburne University of Technology in Melbourne, Australia.
Our board of directors has concluded that Mr. Nathanielsz is well-qualified to serve on our board of directors and has the requisite qualifications, skills and perspectives based on, among other factors, him being a Co-Founder of our Australian company and for his experience in research and development and manufacturing and distribution, as well as him being our controlling stockholder, and his significant business, investment, finance and public company experience, particularly with biotech companies.
| 69 |
Dr. Julian Kenyon ― Dr. Kenyon has served as a director of our Company since inception. Dr. Kenyon co-founded our Australian subsidiary and was appointed as a director of our Australian subsidiary on February 12, 2008. Since 2000, Dr. Kenyon has served as an integrated medical physician and Medical Director of the Dove Clinic for Integrated Medicine in Winchester and London. Dr. Kenyon graduated from the University of Liverpool with a Bachelor of Medicine and Surgery and with a research degree, Doctor of Medicine. Since 1972, he was appointed a Primary Fellow of the Royal College of Surgeons, Edinburgh.
Our board of directors has concluded that Dr. Kenyon is well-qualified to serve on our board of directors and has the requisite qualifications, skills and perspectives based on, among other factors, him being a Co-Founder of our Australian subsidiary and because our business is based on his initial work at the Dove Clinic.
Term of Office
Our directors are appointed for a one-year term to hold office until the next annual general meeting of our stockholders or until removed from office in accordance with our Bylaws and the provisions of the Delaware General Corporation Law. Our directors hold office after the expiration of his or her term until his or her successor is elected and qualified, or until his or her resignation, death or removal in accordance with our Bylaws or the Delaware General Corporation Law.
Our officers are appointed by our board of directors and hold office until removed by our board of directors at any time for any reason.
Family Relationships
There are no family relationships between or among any of our directors or executive officers or persons nominated or chosen by us to become directors or executive officers.
Director Independence
Our board of directors has reviewed the independence of our directors and has determined that none of our directors qualifies as an independent director pursuant to Rule 5605(a)(2) of Nasdaq and applicable SEC rules and regulations. In making this determination, our board of directors considered the relationships that each of our directors has with us and all other facts and circumstances our board of directors deemed relevant in determining their independence.
Board Committees
Our board of directors has no separately designated committees and our two-member board of directors carries out the functions of both an audit committee and a compensation committee. We do not have an audit committee financial expert serving on our board of directors. Due to our limited financial resources, we are not in a position to retain an independent director with the qualifications to serve as an audit committee financial expert at this time.
Scientific Advisory Board
We have a Scientific Advisory Board that provides advice to our management relating to the following:
| ● | The identification, assessment, evaluation, selection, conduct and management of research projects, both those which are under review and are in progress; | |
| ● | Intellectual property; and | |
| ● | Commercialization. |
The Scientific Advisory Board may also address issues related to improving project selection, formal review processes and management procedures within our Company. The Scientific Advisory Board will generally be composed of an advisory panel of clinicians with expertise in translational research.
As of February 14, 2019, the members of the Scientific Advisory Board were:
| ● | Professor John Smyth; | |
| ● | Professor Klaus Kutz (also serving as Chief Medical Officer of the Company); | |
| ● | Dr. Joseph Chalil; |
| 70 |
| ● | Dr. Macarena Perán; | |
| ● | Dr. Juan Antonio Marchal Corrales; and | |
| ● | Dr. Maria Garcia. |
Each of the members of our Scientific Advisory Board acts as an independent consultant and is compensated on an hourly basis for his or her services. There is presently no stock based compensation for their services. In addition, we may have relationships with entities with which the members may be associated.
Professor Kutz is also acting as Chief Medical Officer for Propanc, His compensation continues to be based on an hourly rate as per his Advisory Board Agreement. Propanc intends to appoint Professor Kutz as Chief Medical Officer of Propanc in a full-time capacity at a time that is mutually agreed upon between both parties.
Professor John Smyth ― John Smyth has, for over 25 years, served as Chair of Medical Oncology in the University of Edinburgh Medical School, where his major research interest is the development and evaluation of new anti-cancer drugs. He has published over 300 papers and is Editor-in-Chief of the European Journal of Cancer. He served for several years on the UK Committee on Safety of Medicines, currently Chair’s the Expert Advisory Group for Oncology & Hematology for the Commission on Human Medicines and serves on the Expert Oncology Advisory Group to the European Drug Licensing Board. He is a fellow of the Royal College of Physicians of Edinburgh and London, and fellow of the Royal Society of Edinburgh. He is a past-president of the European Society of Medical Oncology and from 2005 to 2007 was President of the Federation of European Cancer Societies.
Professor Klaus Kutz ―Professor Kutz has over 20 years of experience as an independent consultant in Clinical Pharmacology and Safety for pharmaceutical companies and clinical research organizations. His specialty over the last six years is Oncology, including preparation of multiple NDAs and INDs for small and medium sized pharmaceutical companies. He has prepared, organized and reported clinical Phase I studies in oncology and Phase II studies in different cancer indications (prostate, gastric, ovarian, small cell lung cancer) and Non-Hodgkin Lymphomas. Professor Kutz has more than 13 years of experience as Head of Clinical Pharmacology with world-wide responsibilities for Phase I and Clinical Pharmacokinetics in two internationally operating pharmaceutical companies, setting up and restructuring international Clinical Pharmacology departments. His achievements include the successful world-wide registration of multiple important Sandoz’ compounds by preparation of multiple NDAs (New Drug Applications) and Expert reports (including Written Summary), as well as the preparation of multiple INDs (Investigational New Drug Applications) for Sandoz Pharma Ltd and Sanofi Research. He is a specialist for Internal Medicine, Gastroenterology, and Clinical Pharmacology and he is also Professor of Medicine at the University of Bonn, Germany.
Dr. Joseph Chalil ―Dr. Chalil is a Physician and Executive at Boehringer Ingelheim, the world’s largest privately held pharmaceutical company. Headquartered in Ingelheim, Germany, Boehringer Ingelheim operates globally with 146 affiliates and a more than 47,700 employees. In 2014, Boehringer Ingelheim achieved net sales of about 13.3 billion Euros. Research and development expenditure corresponds to 19.9 percent of its net sales. In addition to his responsibilities at Boehringer Ingelheim, Dr. Chalil is the Chairman of Global Clinical Research and Trial Network of the American Association of Physicians of Indian Origin (AAPI) and has served as Scientific Advisor to AAPI for the past five years. AAPI is the second largest physician organization in the U.S. second only to AMA, and the largest ethnic medical organization in the country. A veteran of the United States Navy Medical Corps, Dr. Chalil is also board certified in healthcare management, and has been awarded Fellowship by the American College of Healthcare Executives, an international professional society of more than 40,000 healthcare executives who lead hospitals, healthcare systems and other healthcare organizations. Dr. Chalil is an expert in U.S. Healthcare policy and a strong advocate for patient centered care and has also served as an advisor to various national political campaigns on healthcare issues. Dr. Chalil completed his higher studies in University of Medicine and Dentistry of New Jersey, Davenport University, JJM Medical College and Baylor College of Medicine. He has been a Visiting Professor at various Universities and serves on various company Boards.
Dr. Macarena Perán ―Dr. Macarena Perán holds a B.S. in Biology and an M.S. in Biochemistry and Molecular Biology from the University of Málaga, Spain. Dr. Perán moved to the Neuroscience Department at Durham University, UK, where she studied the Cellular Distribution and Immobilisation of GABAA Receptors on the cell membrane and graduated in 2000 with a Ph.D. She moved back to Spain and completed another Ph.D. program in the Faculty of Medicine focused on Changes in the Behavior of Central Nervous Proteins; she completed a second Ph.D. from Granada University. In 2005/2006, she attended Bath University, UK, Prof. David Tosh lab, and changed her research interest to the development of new anti-cancer drugs and cell therapy for regenerative medicine. In 2011, she spent a year as a visiting scientist in the Salk Institute for Biological Studies, California, Prof. Juan Carlos Izpisua-Belmonte lab. Currently, Dr. Perán is Reader in Anatomy at University of Jaen in Spain and is working with the Institute for Regenerative Medicine and Pathobiology (IBIMER).
| 71 |
Dr. Juan Antonio Marchal Corrales ―Dr. Juan Antonio Marchal Corrales is Professor of Anatomy and Embryology at the Faculty of Medicine of University of Granada. He graduated in Medicine and Surgery in 1992, obtaining the degree “summa cum laude”. He defended his doctoral thesis in 1996. Prof. Marchal has worked at three universities in different educational categories and is responsible for the research group “Differentiation, Regeneration and Cancer”. He has participated in 39 research projects of national and international character, being principal investigator in 13 of them. He has a total of 145 publications in journals, of which 125 are listed in the Journal Citation Reports. He has spent time at the University of Sassari (Italy) and as visiting professor. He is inventor of 14 patents, 4 of them licensed. He is a member of the Advisory Board of the International Graduate School of the University of Granada, member of the standing committee of the Scientific Council and coordinator of Area Research in the Biosanitary Institute of Granada (ibs.GRANADA) and member of the Governing Board at the Institute of Pathobiology and Regenerative Medicine (IBIMER). He has recently been named director of the Chair Drs. Galera and Requena of Cancer Stem Cell Research at the University of Granada.
Dr. Maria Garcia ―Dr. Maria Garcia, graduated in Biology from University of Granada (Spain) in 1997, became a Molecular Biologist working in the National Centre of Biotechnology characterizing the mechanism of action of “Protein kinase induced by interferon: PKR”. These studies gave rise to a PhD title awarded with an Extraordinary Thesis Award by the Autonomous University of Madrid in 2004. In 2002, Dr. García completed a 3-months stay at the University of Wyoming with Dr. Roth. During the postdoctoral period, she got major public and private funding to characterize new activity of the main tumor suppressor genes that are mutated in more than 50% of human cancers such as p53, ARF and Rb. Dr. García currently has a competitive research contract from the National Health System to lead translational cancer research, aiming at the integration of basic, clinical and epidemiological cancer research in the University Hospital Complex of Granada. She leads a line of research involving new antitumor drugs, biological therapies, biomarkers and cancer stem cell studies. Finally, Dr. García has more than 30 peer-reviewed publications in international journals with an average impact factor of 5 and a H-Index of 14.
Board Leadership Structure
Currently, the office of Acting Chairman of our board of directors and Chief Executive Officer are held by James Nathanielsz. Due to our size and early stage of operations, we believe it is currently most effective to have the Chairman of the board of directors and Chief Executive Officer positions be held by the same individual.
Risk Oversight
Our board of directors will oversee a company-wide approach to risk management. Our board of directors will determine the appropriate risk level for us generally, assess the specific risks faced by us and review the steps taken by management to manage those risks. While our board of directors will have ultimate oversight responsibility for the risk management process, its committees will oversee risk in certain specified areas.
Until we have established our compensation committee of our board of directors, our board of directors will be responsible for overseeing the management of risks relating to our executive compensation plans and arrangements, and the incentives created by the compensation awards it administers. Until we have established our audit committee, our board of directors will oversee management of enterprise risks and financial risks, as well as potential conflicts of interests. Our board of directors will be responsible for overseeing the management of risks associated with the independence of our board of directors.
Code of Ethics
The Board has adopted a Code of Ethics (the “Code”) to apply to all of our directors, officers and employees. The Code is intended to promote ethical conduct and compliance with laws and regulations, to provide guidance with respect to the handling of ethical issues, to implement mechanisms to report unethical conduct, to foster a culture of honesty and accountability, to deter wrongdoing and to ensure fair and accurate financial reporting. A copy of the Code is available at our website www.propanc.com.
Compensation Committee Interlocks and Insider Participation
None of our executive officers currently serves, or in the past three years has served, as a member of the board of directors or compensation committee of another entity that has one or more executive officers serving on our board of directors or the compensation committee. No member of our compensation committee has any other business relationship or affiliation with us other than his or her service as a director.
Section 16(A) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors, executive officers, and persons who own more than 10% of our common stock to file initial reports of ownership and changes in ownership of our common stock and other equity securities with the SEC. These individuals are required by the regulations of the SEC to furnish us with copies of all Section 16(a) forms they file. Based solely on a review of the copies of the forms furnished to us, and written representations from reporting persons that no Forms 5 were required to report delinquent filings, we believe that all filing requirements applicable to our officers, directors and 10% beneficial owners were complied with during the fiscal year ended June 30, 2018.
| 72 |
Nominations to the Board of Directors
General — Our directors take a critical role in guiding our strategic direction and oversee the management of the Company. Our board of directors’ candidates are considered based upon various criteria, such as their broad-based business and professional skills and experiences, a global business and social perspective, concern for the long-term interests of the shareholders, diversity, and personal integrity and judgment. In addition, directors must have time available to devote to our board of directors activities and to enhance their knowledge of our business. Accordingly, we seek to attract and retain highly qualified directors who have sufficient time to attend to their substantial duties and responsibilities to our Company.
Changes to the Procedures by Which Security Holders May Recommend Nominees to Our Board of Directors — During the year ended June 30, 2018, there were no material changes to the procedures by which our security holders may recommend nominees to our board of directors.
Our sole named executive officer, who is our principal executive officer (the “Named Executive Officer”), is Mr. James Nathanielsz, our Chief Executive Officer, Chief Financial Officer, Acting Chairman and a Director.
Summary Compensation Table
The following table sets forth the compensation paid or accrued by us to our Named Executive Officer for the fiscal years ended June 30, 2018 and 2017.
The compensation reported in the summary compensation table below is not necessarily indicative of how we will compensate our sole executive officer in the future. We expect that we will continue to review, evaluate and modify our compensation framework and the compensation of our officer could change as the business develops.
| Year | Salary ($) | Bonus ($) | Option Awards ($) | All
Other Compensation ($) | Total
($) | |||||||||||||||||
| James Nathanielsz(1) | 2018 | $ | 285,277 | (2) | $ | 221,970 | (3) | $ | - | $ | 68,787 | (4) | $ | 576,034 | ||||||||
| Chief Executive Officer and Chief Financial Officer | 2017 | $ | 243,735 | (2) | $ | - | $ | - | $ | 63,716 | (4) | $ | 307,451 | |||||||||
| (1) | For purposes of the information included in the table, the conversion rates as of June 30, 2018 and 2017, $0.7753 and $0.7544, respectively, were used to convert amounts from AUD to USD. | |
| (2) | Under the Nathanielsz Employment Agreement (as defined below), Mr. Nathanielsz received a gross annual salary of $300,000 AUD per year through January 31, 2018. Effective February 1, 2018, the board approved an increase in Mr. Nathanielsz’ gross annual salary to $400,000 AUD. Mr. Nathanielsz has also accrued unused annual leave in the amounts of $20,383 and $17,415 for fiscal years 2018 and 2017, respectively, which are included in the total above. | |
| (3) | On March 16, 2018, the Board granted Mr. Nathanielsz a $300,000 AUD ($221,970 USD) cash bonus for accomplishments while servicing as our chief executive officer, of which $59,226 was paid in the year ended June 30, 2018. | |
| (4) | Under the Nathanielsz Employment Agreement, Mr. Nathanielsz receives a 9.5% contribution to a pension of which he is the beneficiary. In addition, pursuant to the Nathanielsz Employment Agreement, we may make a monthly payment to cover the costs relating to Mr. Nathanielsz use of a vehicle. For fiscal years 2018 and 2017, $41,481 and $40,562, respectively, was paid for use of a vehicle. |
Narrative to Summary Compensation Table
Employment Agreement with James Nathanielsz
The Company and James Nathanielsz entered into an employment agreement as of February 25, 2015 (the “Nathanielsz Employment Agreement”) setting forth the terms and conditions of Mr. Nathanielsz employment as the Company’s President and Chief Executive Officer. The Nathanielsz Employment Agreement also contemplates that Mr. Nathanielsz serves as a member of the Board. The Nathanielsz Employment Agreement expires February 25, 2018; however, the term of the Nathanielsz Employment Agreement automatically renews for successive one-year periods unless either party provides 30 days’ prior written notice of its intent not to renew. As of the date of this prospectus, the Nathanielsz Employment Agreement was automatically renewed for a one-year period.
| 73 |
The Nathanielsz Employment Agreement provides Mr. Nathanielsz with a base salary of $25,000 AUD per month ($300,000 AUD annually) and a monthly contribution to Mr. Nathanielsz’s pension equal to 9.5% of his monthly salary. Mr. Nathanielsz has the ability to convert any accrued but unpaid salary into common stock at the end of each fiscal year at a conversion price to be determined by Mr. Nathanielsz and the Company, which will in no event be lower than par value or higher than the closing bid price on the date of conversion .The Company has also agreed to pay Mr. Nathanielsz an annual discretionary bonus in an amount up to 200% of his annual base salary, which bonus shall be determined by the Board and based upon the performance of the Company.
Mr. Nathanielsz is entitled to 20 days of annual leave and 8 days of paid sick leave. Mr. Nathanielsz is also entitled to participate in employee benefits plans, fringe benefits and perquisites maintained by the Company to the extent the Company provides similar benefits or perquisites (or both) to similarly situated executives of the Company.
In the event that the Company provides notice of non-renewal of the Nathanielsz Employment Agreement, the Company terminates Mr. Nathanielsz without cause (as defined in the Nathanielsz Employment Agreement) or Mr. Nathanielsz terminates his employment for good reason (as defined in the Nathanielsz Employment Agreement), the Company has agreed to pay Mr. Nathanielsz a severance payment in an amount equal to Mr. Nathanielsz’s base salary for the year of termination in addition to accrued but unpaid salary, reimbursement of expenses and certain other employee benefits as determined under the terms of the applicable plans (“Accrued Amounts”). In the event that Mr. Nathanielsz provides notice of non-renewal of the Nathanielsz Employment Agreement, the Company terminates Mr. Nathanielsz for cause or Mr. Nathanielsz terminates his employment without good reason, Mr. Nathanielsz is only entitled to the Accrued Amounts.
The Company has agreed to indemnify Mr. Nathanielsz for any liabilities, costs and expenses incurred in the event that he is made a party to a proceeding due to his roles with the Company, other than any proceeding initiated by Mr. Nathanielsz or the Company relating to any dispute with respect to the Nathanielsz Employment Agreement or Mr. Nathanielsz’s employment.
Under the terms of the Nathanielsz Employment Agreement, Mr. Nathanielsz is also subject to certain restrictive covenants, including a one-year non-compete.
On April 14, 2016, the Board approved Amendment No.1 to the Nathanielsz Employment Agreement to include a provision pursuant to which the Company pays Mr. Nathanielsz a monthly amount to cover the costs relating to Mr. Nathanielsz use of a vehicle.
Also on April 14, 2016, the Board approved the payment of an annual bonus to the Chief Executive Officer based on certain performance achievements in 2015 in accordance with the terms of the Nathanielsz Employment Agreement. The bonus amount approved was $200,000 AUD (or 66.66% of the CEO’s current base salary).
On April 14, 2016 (the “Grant Date”), the Board of Directors of the Company granted 286,000 stock options with an exercise price of $7.50 per share (market value of the Company’s common stock on the Grant Date), to Mr. Nathanielsz. 95,333 of such stock options vested on April 14, 2016, 95,333 of such stock options vest on April 14, 2017 (the first anniversary of the Grant Date) and 95,334 of such stock options shall vest on April 14, 2018 (the second anniversary of the Grant Date). These stock options expire on April 14, 2021. The fair value of the 286,000 options at the Grant Date is $1,962,440.
On August 15, 2016, the Board granted Mr. Nathanielsz a cash bonus in the amount of $250,000 USD (representing 83.33% of his annual base salary), of which $130,000 was paid in the year ended June 30, 2017. An additional $50,000 of this bonus was paid in the current fiscal year, pursuant to the terms of the Nathanielsz Employment Agreement, based upon the performance of the Company.
On September 25, 2017, the Company and Mr. Nathanielsz entered into an amendment to the Nathanielsz Employment Agreement. The amendment provides that the annual leave section of the Nathanielsz Employment Agreement be changed to permit any unused annual leave to roll over from year-to-year and that Mr. Nathanielsz would be entitled to receive any accrued but unpaid annual leave in the event of the termination of his employment. The Employment Agreement also acknowledges that Mr. Nathanielsz has accrued $121,884 of unused annual leave since he joined the Company in 2007. These amended provisions are intended to make the Nathanielsz Employment Agreement consistent with Australian law governing employee leave. In addition, the amendment clarifies certain activities that Mr. Nathanielsz is prohibited from engaging in while employed at the Company in order to prevent competitive harm.
On March 16, 2018, the Board approved an increase of AU$100,000 (US$77,328.33) in Mr. Nathanielsz’ annual base salary, from AU$300,000 (US$231,984.99) to AU$400,000 (US$309,313.32), effective immediately. In addition, having reviewed the Company’s corporate objectives and performance criteria, including performance goals for Mr. Nathanielsz, the Board awarded a cash bonus of AU$300,000 (US$231,984.99) to Mr. Nathanielsz, which is equal to 100% of his annual base salary in 2017, and is consistent with the bonus parameters set forth in Mr. Nathanielsz’s existing employment agreement with Company.
| 74 |
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth certain information with respect to grants of plan-based awards for the fiscal year ended June 30, 2018 to the Named Executive Officer. Except as set forth below, all of the outstanding equity awards granted to our Named Executive Officer were fully vested as of June 30, 2018.
| Option awards | Stock awards | |||||||||||||||||||||
| Name | Number
of Securities Underlying Unexercised Options (#) Exercisable | Number
of Securities Underlying Unexercised Options (#) Unexercisable | Option ($) | Option Expiration Date | Number
of Shares, Units or Other Rights That Have Not Vested (#) | Market Value or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) | ||||||||||||||||
| James Nathanielsz(1) | 286,000 | - | $ | 7.50 | April 14, 2021 | - | - | |||||||||||||||
| (1) | On April 14, 2016, the Board granted Mr. Nathanielsz 286,000 stock options at an exercise price of $7.50 per share (market value of the common stock on the Grant Date). 95,333 of such stock options vested on April 14, 2016 and expire on April 14, 2021, 95,333 of such stock options vested on April 14, 2017 (the first anniversary of the Grant Date) and expire on April 14, 2021 and 95,334 of such stock options vested on April 14, 2018 (the second anniversary of the Grant Date) and expire on April 14, 2021. The fair value of the 286,000 options at the Grant Date is $1,962,440. |
Director Compensation for the Fiscal Year Ended June 30, 2018
| Name | Fees
earned or paid in cash ($) | Option Awards ($) | All
Other Compensation ($) | Total
($) | ||||||||||||
| Dr. Julian Kenyon (1) | $ | 41,866 | (2) | - | - | $ | 41,866 | |||||||||
| (1) | For purposes of the information included in the table, the conversion rate as of June 30, 2018, $0.7753 was used to convert amounts from AUD to USD. | |
| (2) | Under the Director Agreement (defined below), Dr. Kenyon received a gross consideration of $10,000 AUD per month through September 2016. Effective October 2016 Dr. Kenyon receives gross monthly compensation of $4,500 AUD or $3,489 USD per month for his services as a director of our Company. See “Compensation of Directors — Director Agreement with Dr. Julian Kenyon” below for additional details. |
Director Agreement with Dr. Julian Kenyon
The Director Agreement sets forth the terms and conditions of Dr. Kenyon’s service as a director on the Board (the “Director Agreement”). Dr. Kenyon’s appointment term was originally for three years, expiring on February 25, 2018; however, this term automatically renews for successive one-year periods unless either party provides 30 days’ prior written notice of its intent not to renew. As of the date of this prospectus, the Director Agreement was automatically renewed for a one-year period.
Under the Director Agreement, Dr. Kenyon received monthly consideration of $10,000 AUD ($120,000 AUD annualized). Dr. Kenyon has the ability to convert any accrued but unpaid compensation into common stock at the end of each fiscal year at a conversion price to be determined by Dr. Kenyon and the Company, which will in no event be lower than par value or higher than the closing bid price on the date of conversion.
In the event that the Company provides notice of non-renewal of the Director Agreement, the Company terminates Dr. Kenyon without cause (as defined in the Director Agreement) or Dr. Kenyon terminates his services to the Company for good reason (as defined in the Director Agreement), the Company agreed to pay Dr. Kenyon a severance payment in an amount equal to Dr. Kenyon’s base salary for the year of termination in addition to accrued but unpaid salary and reimbursement of expenses (“Kenyon Accrued Amounts”). In the event that Dr. Kenyon provides notice of non-renewal of the Director Agreement, the Company terminates Dr. Kenyon for cause or Dr. Kenyon terminates his services without good reason, Dr. Kenyon is only entitled to the Kenyon Accrued Amounts.
The Company has agreed to indemnify Dr. Kenyon for any liabilities, costs and expenses incurred in the event that he is made a party to a proceeding due to his role with the Company, other than any proceeding initiated by Dr. Kenyon or the Company relating to any dispute with respect to the Director Agreement or Dr. Kenyon’s service as a director.
| 75 |
Under the terms of the Director Agreement, Dr. Kenyon is also subject to certain restrictive covenants, including a one-year non-compete.
On April 14, 2016 (the “Grant Date”), the board of directors of the Company granted 286,000 stock options with an exercise price of $7.50 per share (market value of the Company’s common stock on the Grant Date), to Dr. Kenyon. 95,333 of such stock options vested on April 14, 2016 and expire on April 14, 2021, 95,333 of such stock options vested on April 14, 2017 (the first anniversary of the Grant Date) and expire on April 14, 2021 and 95,334 of such stock options vested on April 14, 2018 (the second anniversary of the Grant Date) and expire on April 14, 2021. The fair value of the 286,000 options at the Grant Date was $1,962,440.
Effective October 2016, Dr. Kenyon receives gross monthly compensation of $4,500 and on $3,489 USD per month.
Other Director Compensation
Directors are reimbursed for reasonable expenses incurred in attending meetings and carrying out duties as board members.
Scientific Advisory Board Members Compensation
The Company has entered into Scientific Advisory Board Member Agreements with certain members of its Scientific Advisory Board (the “SAB Agreements”). The SAB Agreements contain substantially similar terms and primarily relate to the protection of the Company’s intellectual property. The SAB Agreements also include provisions for the members’ compensation for the services performed as a member of the Scientific Advisory Board. Messrs. Kutz, Brandt and Smyth each are paid a monetary fee for each year of service provided.
Narrative Disclosure of Compensation Policies and Practices as They Relate to Our Risk Management
We believe that our compensation policies and practices for all employees and other individual service providers, including executive officers, do not create risks that are reasonably likely to have a material adverse effect on us.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information regarding beneficial ownership of our capital stock by:
| ● | each person, or group of affiliated persons, known by us to beneficially own more than 5% of our common stock; | |
| ● | each of our directors; | |
| ● | our sole Named Executive Officer; and | |
| ● | all of our current executive officers and directors as a group. |
The following table is based upon information supplied by to us by our officers, directors and certain principal stockholders. We have determined beneficial ownership in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. In addition, the rules include shares of common stock that the person has the right to acquire beneficial ownership within 60 days, including common stock issuable pursuant to the exercise of options that are either immediately exercisable or exercisable on or before April 15, 2019, which is 60 days after February 14, 2019. These shares are deemed to be outstanding and beneficially owned by the person holding those options for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them, subject to applicable community property laws.
Except as otherwise noted below, the address for each person or entity listed in the table is c/o Propanc Biopharma, Inc., 302, 6 Butler Street, Camberwell, VIC, 3124 Australia.
| 76 |
| Common
Stock Beneficially Owned | Series
A Preferred Stock Beneficially Owned | Series
B Preferred Stock Beneficially Owned | ||||||||||||||||||||||
| Name and Address of Beneficial Owner | Number of Shares Beneficially Owned | Percentage
of Class(1) | Number of Shares Beneficially Owned | Percentage
of Class(2) | Number of Shares Beneficially Owned | Percentage
of Class (2) | ||||||||||||||||||
| North Horizon Pty Ltd.(3) | 170,844 | * | 500,000 | 100 | % | - | - | |||||||||||||||||
| James Nathanielsz(4) | 456,844 | * | - | - | 1 | 100 | % | |||||||||||||||||
| Dr. Julian Kenyon(5) | 399,870 | * | - | - | - | - | ||||||||||||||||||
| All directors and executive officers, as a group (2 persons) | 856,715 | * | 500,000 | 100 | % | 1 | 100 | % | ||||||||||||||||
* Represents beneficial ownership of less than one percent.
(1) Applicable percentages are based on 333,896,577 shares of our common stock outstanding as of February 14, 2019.
(2) Applicable percentages are based on 500,000 shares of our Series A Preferred Stock and 1 share of our Series B Preferred Stock outstanding as of February 14, 2019, except where the person or entity has the right to receive shares within the next 60 days, which would increase the number of shares owned by such person or entity and the number of shares outstanding.
(3) North Horizon Pty Ltd. is a Nathanielsz Family Trust. Mr. James Nathanielsz, the Chief Executive Officer, Chief Financial Officer and a director of our Company, has voting and investment power over these shares.
(4) Represents 170,844 shares of our common stock held by North Horizon Pty Ltd. and 286,000 shares of common stock issuable to Mr. Nathanielsz pursuant to his stock options currently exercisable or exercisable within 60 days of February14, 2019.
(5) Represents 113,870 shares of our common stock held by Dr. Julian Kenyon, a director of our Company, and 286,000 shares of our common stock issuable to Dr. Kenyon pursuant to his stock options currently exercisable or exercisable within 60 days of February 14, 2019.
TRANSACTIONS WITH RELATED PERSONS, PROMOTERS AND CERTAIN CONTROL PERSONS AND
DIRECTOR INDEPENDENCE
The following includes a summary of transactions since July 1, 2017 to which we have been a party, in which the amount involved in the transaction exceeded $120,000, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than 5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest, other than equity and other compensation, termination, change in control and other arrangements, which are described above under “Executive Compensation.”
Effective May 5, 2016, we entered into an agreement for the lease of our principal executive offices with North Horizon Pty Ltd, of which Mr. Nathanielsz and his wife are the owners and directors. The lease has a five-year term and provides for annual rental payments of $39,600 AUD, which includes $3,600 of goods and service tax, for total payments of $198,000 AUD during the term of the lease.
Mr. Nathanielsz’s wife, Sylvia Nathanielsz, is and has been an employee of our Company since October 2015. Mrs. Nathanielsz receives an annual salary of $57,570 AUD, or $44,634 USD, and is entitled to benefits customarily expected to be provided to employees of the Company. From July 2015 until October 2015, Mrs. Nathanielsz was an independent contractor serving our Company and was paid approximately $13,632 for her services.
Employment and Director Compensation Arrangements
The relationships and related party transactions described herein are in addition to any employment and director compensation arrangements with our executive officers and directors, which are described above under “Executive Compensation — Narrative to Summary Compensation Table and Director Compensation.”
| 77 |
Indemnification Agreements
Our Certificate of Incorporation provides that none of our officers or directors shall be personally liable for any obligations of our Company or for any duties or obligations arising out of any acts or conduct of said officer or director performed for or on behalf of our Company, including without limitation, acts of negligence or contributory negligence. In addition, our Certificate of Incorporation provides that we shall indemnify and hold harmless each person and their heirs and administrators who shall serve at any time hereafter as a director or officer of our Company from and against any and all claims, judgments and liabilities to which such persons shall become subject by reason of their having heretofore or hereafter been a director or officer of our Company, or by reason of any action alleged to have heretofore or hereafter taken or omitted to have been taken by him or her as such director or officer, and that we shall reimburse each such person for all legal and other expenses reasonably incurred by him or her in connection with any such claim, judgment or liability, including our power to defend such persons from all suits or claims as provided for under the provisions of the General Corporation Law of the State of Delaware; provided, however, that no such persons shall be indemnified against, or be reimbursed for, any expense incurred in connection with any claim or liability arising out of his (or her) own willful misconduct. Our Certificate of Incorporation also provide that we are obligated to advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding, and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in that capacity regardless of whether we would otherwise be permitted to indemnify him or her under the provisions of Delaware law. In addition, we intend to enter into indemnification agreements with our directors and officers and some of our executives may have certain indemnification rights arising under their employment agreements with us. We believe that these provisions of our Certificate of Incorporation and indemnification agreements are necessary to attract and retain qualified persons as directors and officers.
The limitation of liability and indemnification provisions in our Certificate of Incorporation may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against our directors and officers, even though an action, if successful, might benefit us and our stockholders. A stockholder’s investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
Policies and Procedures for Transactions with Related Persons
We intend to adopt a written related-person transactions policy that will set forth our policies and procedures regarding the identification, review, consideration and oversight of “related-person transactions.” For purposes of this policy only, a “related-person transaction” shall be a transaction, arrangement or relationship (or any series of similar transactions, arrangements or relationships) in which we and any “related person” are participants involving an amount that exceeds $120,000.
Director Independence
Our board of directors has reviewed the independence of our directors and has determined that none of our directors qualifies as an independent director pursuant to Rule 5605(a)(2) of Nasdaq and applicable SEC rules and regulations. In making this determination, our board of directors considered the relationships that each of our directors has with us and all other facts and circumstances our board of directors deemed relevant in determining their independence.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports and other information with the SEC. Such filings are available to the public over the Internet at the SEC’s website at http://www.sec.gov.
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the securities offered under this prospectus. This prospectus, which forms a part of that registration statement, does not contain all information included in the registration statement. Certain information is omitted and you should refer to the registration statement and its exhibits.
You may review a copy of the registration statement, and the reports and other information that we file with the SEC, at the SEC’s public reference room at 100 F Street, N.E. Washington, D.C. 20549 on official business days during the hours of 10 a.m. to 3 p.m. You may obtain information on the operation of the public reference room by calling the SEC at 1-800-SEC-0330. You may also read and copy any materials we file with the SEC at the SEC’s public reference room. Our filings and the registration statement can also be reviewed by accessing the SEC’s website at http://www.sec.gov.
Statements contained in this prospectus as to the contents of any contract or other document that we have filed as an exhibit to the registration statement are qualified in their entirety by reference to the exhibits for a complete statement of their terms and conditions.
The representations, warranties and covenants made by us in any agreement that is filed as an exhibit to the registration statement of which this prospectus is a part were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were made as of an earlier date. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
| 78 |
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Our Certificate of Incorporation contains provisions that limit the liability of our directors for monetary damages to the fullest extent permitted by Delaware law. Consequently, our directors will not be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duties as directors, except liability for:
| ● | any breach of the director’s duty of loyalty to us or our stockholders; | |
| ● | any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; | |
| ● | unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the General Corporation Law of the State of Delaware; or | |
| ● | any transaction from which the director derived an improper personal benefit. |
Our Certificate of Incorporation, as amended, provides that we are required to indemnify our directors and officers, in each case to the fullest extent permitted by Delaware law. Our Certificate of Incorporation also provides that we are obligated to advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding, and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in that capacity regardless of whether we would otherwise be permitted to indemnify him or her under the provisions of Delaware law.
To the extent that indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling our company pursuant to the foregoing provisions, we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
| 79 |
INDEX TO FINANCIAL STATEMENTS
| Unaudited Interim Financial Statements for the Three and Six Months Ended December 31, 2018 | |
| Page | |
| Condensed Consolidated Balance Sheets as of December 31, 2018 (unaudited) and June 30, 2018 | F-2 |
| Condensed Consolidated Statements of Operations and Comprehensive Income (Loss) for the three and six months ended December 31, 2018 and 2017 (unaudited) | F-3 |
| Condensed Consolidated Statements of Cash Flows for the six months ended December 31, 2018 and 2017 (unaudited) | F-4 |
| Notes to Consolidated Financial Statements (unaudited) | F-5 - F-34 |
Audited Consolidated Financial Statements for the Fiscal Years Ended June 30, 2018 and 2017
| F-1 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
CONDENSED CONSOLIDATED BALANCE SHEETS
| December 31, 2018 | June 30, 2018 | |||||||
| (Unaudited) | ||||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | $ | 124,538 | $ | 19,921 | ||||
| GST tax receivable | 8,639 | 6,257 | ||||||
| Prepaid expenses and other current assets | 254,878 | 34,712 | ||||||
| TOTAL CURRENT ASSETS | 388,055 | 60,890 | ||||||
| Deferred offering costs | 313,923 | - | ||||||
| Security deposit - related party | 2,114 | 2,220 | ||||||
| Property and equipment, net | 8,505 | 8,277 | ||||||
| TOTAL ASSETS | $ | 712,597 | $ | 71,387 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable | $ | 1,062,609 | $ | 1,157,369 | ||||
| Accrued expenses and other payables | 296,922 | 364,404 | ||||||
| Convertible notes and related accrued interest, net of discounts and premiums | 2,798,624 | 4,699,299 | ||||||
| Embedded conversion option liabilities | 66,490 | 371,532 | ||||||
| Due to directors - related parties | 31,329 | 32,898 | ||||||
| Loans from directors and officer - related parties | 52,140 | 54,753 | ||||||
| Employee benefit liability | 308,519 | 143,052 | ||||||
| TOTAL CURRENT LIABILITIES | 4,616,633 | 6,823,307 | ||||||
| Commitments and Contingencies (See Note 7) | ||||||||
| STOCKHOLDERS’ DEFICIT: | ||||||||
| Preferred stock, 1,500,005 shares authorized, $0.01 par value: | ||||||||
| Series A preferred stock, $0.01 par value; 500,000 shares authorized; 500,000 and 500,000 shares issued and outstanding as of December 31, 2018 and June 30, 2018, respectively | 5,000 | 5,000 | ||||||
| Series B preferred stock, $0.01 par value; 5 shares authorized; 1 and 1 share issued and outstanding as of December 31, 2018 and June 30, 2018, respectively | - | - | ||||||
| Common stock, $0.001 par value; 4,000,000,000 shares authorized; 273,276,784 and 46,429,423 shares issued, and 273,252,306 and 46,404,945 shares outstanding as of December 31, 2018 and June 30, 2018, respectively | 273,277 | 46,429 | ||||||
| Additional paid-in capital | 43,729,392 | 38,167,877 | ||||||
| Accumulated other comprehensive income (loss) | 992,424 | 357,929 | ||||||
| Accumulated deficit | (48,857,652 | ) | (45,282,678 | ) | ||||
| Treasury stock (24,478 shares) | (46,477 | ) | (46,477 | ) | ||||
| TOTAL STOCKHOLDERS’ DEFICIT | (3,904,036 | ) | (6,751,920 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | $ | 712,597 | $ | 71,387 | ||||
The accompanying unaudited condensed notes are an integral part of these unaudited condensed consolidated financial statements.
| F-2 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME (LOSS)
(Unaudited)
| Three
Months Ended December 31, | Six
Months Ended December 31, | |||||||||||||||
| 2018 | 2017 | 2018 | 2017 | |||||||||||||
| REVENUE | ||||||||||||||||
| Revenue | $ | - | $ | - | $ | - | $ | - | ||||||||
| OPERATING EXPENSES | ||||||||||||||||
| Administration expenses | 656,594 | 395,442 | 1,041,194 | 1,026,848 | ||||||||||||
| Occupancy expenses | 6,449 | 6,899 | 14,527 | 15,729 | ||||||||||||
| Research and development | 94,777 | 1,034,729 | 150,970 | 1,598,468 | ||||||||||||
| TOTAL OPERATING EXPENSES | 757,820 | 1,437,070 | 1,206,691 | 2,641,045 | ||||||||||||
| LOSS FROM OPERATIONS | (757,820 | ) | (1,437,070 | ) | (1,206,691 | ) | (2,641,045 | ) | ||||||||
| OTHER INCOME (EXPENSE) | ||||||||||||||||
| Interest expense | (494,989 | ) | (486,754 | ) | (1,119,527 | ) | (1,379,186 | ) | ||||||||
| Interest income | 23 | 39 | 27 | 67 | ||||||||||||
| Change in fair value of derivative liabilities | 39,470 | (260,045 | ) | (1,931,646 | ) | (229,771 | ) | |||||||||
| Gain (loss) on debt settlements, net | (132 | ) | 28,244 | 14,289 | 36,814 | |||||||||||
| Gain (loss) on extinguishment of debt, net | (20,355 | ) | (83,727 | ) | 1,165,516 | (83,727 | ) | |||||||||
| Foreign currency transaction loss | (333,205 | ) | (142,944 | ) | (613,912 | ) | (9,152 | ) | ||||||||
| TOTAL OTHER INCOME (EXPENSE) | (809,188 | ) | (945,187 | ) | (2,485,253 | ) | (1,664,955 | ) | ||||||||
| LOSS BEFORE TAXES | (1,567,008 | ) | (2,382,257 | ) | (3,691,944 | ) | (4,306,000 | ) | ||||||||
| TAX BENEFIT | 116,970 | 180,278 | 116,970 | 180,278 | ||||||||||||
| NET LOSS | $ | (1,450,038 | ) | $ | (2,201,979 | ) | $ | (3,574,974 | ) | $ | (4,125,722 | ) | ||||
| BASIC AND DILUTED NET LOSS PER SHARE | $ | (0.08 | ) | $ | (0.20 | ) | $ | (0.02 | ) | $ | (0.49 | ) | ||||
| BASIC AND DILUTED WEIGHTED AVERAGE SHARES OUTSTANDING | 17,540,299 | 10,955,901 | 154,556,976 | 8,399,687 | ||||||||||||
| NET LOSS | $ | (1,450,038 | ) | $ | (2,201,979 | ) | $ | (3,574,974 | ) | $ | (4,125,722 | ) | ||||
| OTHER COMPREHENSIVE INCOME (LOSS) | ||||||||||||||||
| Unrealized foreign currency translation gain (loss) | 358,279 | 19,329 | 634,495 | (170,264 | ) | |||||||||||
| TOTAL OTHER COMPREHENSIVE INCOME (LOSS) | 358,279 | 19,329 | 634,495 | (170,264 | ) | |||||||||||
| TOTAL COMPREHENSIVE INCOME (LOSS) | $ | (1,091,759 | ) | $ | (2,182,650 | ) | $ | (2,940,479 | ) | $ | (4,295,986 | ) | ||||
The accompanying unaudited condensed notes are an integral part of these unaudited condensed consolidated financial statements.
| F-3 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
| Six Months Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (3,574,974 | ) | $ | (4,125,722 | ) | ||
| Adjustments to Reconcile Net Loss to Net Cash Used in Operating Activities: | ||||||||
| Issuance and amortization of common stock for services | 69,000 | 40,419 | ||||||
| Issuance of convertible promissory notes for services | - | 310,000 | ||||||
| (Gain) loss on settlements, net | (14,289 | ) | (36,814 | ) | ||||
| Foreign currency transaction loss (gain) | 613,912 | 9,152 | ||||||
| Depreciation expense | 1,068 | 1,128 | ||||||
| Amortization of debt discounts | 303,813 | 400,284 | ||||||
| Change in fair value of derivative liabilities | 1,931,646 | 229,771 | ||||||
| Gain on extinguishment of debt | (1,237,285 | ) | 83,727 | |||||
| Stock option expense | - | 329,761 | ||||||
| Accretion of put premium | 699,230 | 876,462 | ||||||
| Changes in Assets and Liabilities: | ||||||||
| GST receivable | (2,757 | ) | 3,862 | |||||
| Prepaid expenses and other assets | (53,104 | ) | 4,897 | |||||
| Accounts payable | (26,388 | ) | 952,931 | |||||
| Employee benefit liability | 177,232 | 12,160 | ||||||
| Accrued expenses | (51,533 | ) | (265,568 | ) | ||||
| Accrued interest | 107,643 | 102,224 | ||||||
| NET CASH USED IN OPERATING ACTIVITIES | (1,056,786 | ) | (1,071,326 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Purchase of equipment | (1,662 | ) | - | |||||
| NET CASH USED IN INVESTING ACTIVITIES | (1,662 | ) | - | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Proceeds from convertible promissory notes | 1,054,089 | 1,274,000 | ||||||
| Repayments of convertible promissory notes | (219,000 | ) | - | |||||
| Proceeds from the issuance of common stock | 405,773 | - | ||||||
| Fees associated with offering costs | (15,000 | ) | - | |||||
| Proceeds from the exercise of warrants | 30 | - | ||||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 1,225,892 | 1,274,000 | ||||||
| Effect of exchange rate changes on cash | (62,827 | ) | (159,221 | ) | ||||
| NET INCREASE IN CASH | 104,617 | 43,453 | ||||||
| CASH AT BEGINNING OF PERIOD | 19,921 | 69,043 | ||||||
| CASH AT END OF PERIOD | $ | 124,538 | $ | 112,496 | ||||
| Supplemental Disclosure of Cash Flow Information | ||||||||
| Cash paid during the year: | ||||||||
| Interest | $ | 8,585 | $ | - | ||||
| Income Tax | $ | - | $ | - | ||||
| Supplemental Disclosure of Non-Cash Investing and Financing Activities | ||||||||
| Reduction of put premium related to conversions of convertible notes | $ | 1,308,393 | $ | 337,437 | ||||
| Conversion of convertible notes and accrued interest to common stock | $ | 2,508,417 | $ | 1,377,928 | ||||
| Proceeds receivable from the issuance of common stock | $ | 168,787 | $ | - | ||||
| Deferred financing costs associated with equity purchase agreement | $ | 298,923 | $ | - | ||||
| Discounts related to derivative liability | $ | 50,000 | $ | 310,000 | ||||
The accompanying unaudited condensed notes are an integral part of these unaudited condensed consolidated financial statements.
| F-4 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
NOTE 1 – NATURE OF OPERATIONS AND SUMMARY OF SIGNIFICANT ACCOUNTING AND REPORTING POLICIES
Nature of Operations
Propanc Biopharma, Inc. (the “Company,” “we,” “us” or “our”) was originally incorporated in Melbourne, Victoria Australia on October 15, 2007 as Propanc PTY LTD, and continues to be based in Camberwell, Victoria Australia. Since its inception, substantially all of the operations of the Company have been focused on the development of new cancer treatments targeting high-risk patients, particularly cancer survivors, who need a follow-up, non-toxic, long-term therapy designed to prevent the cancer from returning and spreading. The Company anticipates establishing global markets for its technologies. Our lead product candidate, which we refer to as PRP, is an enhanced pro-enzyme formulation designed to enhance the anti-cancer effects of multiple enzymes acting synergistically. It is currently in the preclinical phase of development.
On November 23, 2010, the Company was incorporated in the state of Delaware as Propanc Health Group Corporation. In January 2011, to reorganize the Company, we acquired all of the outstanding shares of Propanc PTY LTD on a one-for-one basis making it a wholly-owned subsidiary of the Company.
On July 22, 2016, the Company formed a wholly owned subsidiary, Propanc (UK) Limited under the laws of England and Wales for the purpose of submitting an orphan drug application to the European Medicines Agency as a small and medium-sized enterprise. As of December 31, 2018, there has been no activity within this entity.
Effective April 20, 2017, the Company changed its name to “Propanc Biopharma, Inc.” to better reflect the Company’s stage of operations and development.
The Company has filed multiple patent applications relating to its lead product, PRP. The first application was filed in October 2010 in each of the countries listed in the table below. This application has been granted and remains in force in the United States, Belgium, Czech Republic, Denmark, France, Germany, Ireland, Italy, Netherlands, Portugal, Spain, Sweden, Switzerland, Liechtenstein, Turkey, United Kingdom, Australia, China, Japan, Indonesia, Israel, New Zealand, Singapore, Malaysia, South Africa, Mexico, Republic of Korea and India. In Brazil and Canada, the patent application remains under examination.
In 2016 and 2017 we filed other patent applications, as indicated below. Three applications were filed under the Patent Cooperation Treaty (the “PCT”). The PCT assists applicants in seeking patent protection by filing one international patent application under the PCT, which allows the applicants to seek protection for an invention in over 150 countries. Once national or regional applications are filed, the application is placed under the control of the national or regional patent offices, as applicable, in what is called the national or regional phase. One PCT application, filed in November 2016, entered the national phase in July 2018 in each of the countries listed in the table below. A second application filed in January 2017 entered the national phase commencing July 2018. A third application entered the national phase in October 2018.
| F-5 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
| No. | Title | Country | Case Status | Date Filed | ||||
| 1. | A pharmaceutical composition for treating cancer comprising trypsinogen and/or chymotrypsinogen and an active agent selected from a selenium compound, a vanilloid compound and a cytoplasmic reduction agent. | USA, Belgium, Czech Republic, Denmark, France, Germany, Ireland, Italy, Netherlands, Portugal, Spain, Sweden, Switzerland, Liechtenstein, Turkey, United Kingdom, Australia, China, Japan, Indonesia, Israel, New Zealand, Malaysia, Singapore, Malaysia South Africa, Mexico, Republic of Korea and India |
Granted
|
Oct-22-2010 | ||||
Brazil and Canada |
Under Examination Divisional applications filed and under examination in USA, Mexico and China |
|||||||
| 2. | Proenzyme composition | Australia, Canada, China, Europe, Hong Kong, India, Indonesia, Israel, Japan, Malaysia, New Zealand, Singapore, South Africa and USA | Application filed and pending | Nov-11-2016 | ||||
| 3. | Cancer Treatment | Australia, Canada, China, Europe, Hong Kong, Israel, Japan, Malaysia, New Zealand, Singapore and USA | Application filed and pending | Jan-27-2017 | ||||
| 4. | Composition of proenzymes for cancer treatment | Australia, China, Europe, Japan and USA | Application filed and pending | Apr-12-2017 |
The Company hopes to capture and protect additional patentable subject matter based on the Company’s field of technology relating to pharmaceutical compositions of proenzymes for treating cancer by filing additional patent applications as it advances its lead product candidate, PRP, through various stages of development.
Increase in Authorized Common Stock
On September 21, 2018, the Company filed a Certificate of Incorporation to increase the number of authorized shares of the Company’s common stock from 400,000,000 to 4,000,000,000, which was approved by the Company’s board of directors and holders of a majority of the Company’s voting stock on August 28, 2018.
Basis of Presentation
The Company’s interim unaudited condensed consolidated financial statements included in this Quarterly Report on Form 10-Q (this “Quarterly Report”) have been prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”) and pursuant to the rules and regulations of the U.S. Securities and Exchange Commission (“SEC”). In the opinion of the Company’s management, all adjustments (consisting of normal recurring adjustments and reclassifications and non-recurring adjustments) necessary to present fairly our results of operations for the three and six months ended December 31, 2018 and 2017 and cash flows for the six months ended December 31, 2018 and 2017 and our financial position at December 31, 2018 have been made. The Company’s results of operations for the three and six months ended December 31, 2018 are not necessarily indicative of the operating results to be expected for the full year.
Reference is frequently made herein to the Financial Accounting Standards Board (the “FASB”) Accounting Standards Codification (“ASC”). This is the source of authoritative US GAAP recognized by the FASB to be applied to non-governmental entities. Each ASC reference in this Quarterly Report is presented with a three-digit number, which represents its Topic. As necessary for explanation and as applicable, an ASC topic may be followed with a two-digit subtopic, a two-digit section or a two-or-three-digit paragraph.
Certain information and disclosures normally included in the notes to the Company’s annual audited consolidated financial statements have been condensed or omitted from the Company’s interim unaudited condensed consolidated financial statements included in this Quarterly Report. Accordingly, these interim unaudited condensed consolidated financial statements should be read in conjunction with the Company’s audited consolidated financial statements and notes thereto for the fiscal year ended June 30, 2018. The June 30, 2018 balance sheet is derived from those statements.
| F-6 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
Principles of Consolidation
The condensed consolidated financial statements include the accounts of Propanc Biopharma, Inc., the parent entity, and its wholly-owned subsidiary, Propanc PTY LTD. All inter-company balances and transactions have been eliminated in consolidation. Propanc (UK) Limited was an inactive subsidiary at December 31, 2018.
Use of Estimates
The preparation of financial statements in conformity with the accounting principles generally accepted in the United States of America (“US GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates. Significant estimates in the accompanying unaudited condensed consolidated financial statements include the estimates of useful lives for depreciation, valuation of derivatives, valuation of beneficial conversion features on convertible debt, allowance for uncollectable receivables, valuation of equity based instruments issued for other than cash, the valuation allowance on deferred tax assets and foreign currency translation due to certain average exchange rates applied in lieu of spot rates on transaction dates.
Foreign Currency Translation and Other Comprehensive Income (Loss)
The Company’s functional currency is the Australian dollar (AUD). For financial reporting purposes, the Australian dollar has been translated into United States dollars ($) and/or (USD) as the reporting currency. Assets and liabilities are translated at the exchange rate in effect at the balance sheet date. Revenues and expenses are translated at the average rate of exchange prevailing during the reporting period. Equity transactions are translated at each historical transaction date spot rate. Translation adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’ equity (deficit) as “Accumulated other comprehensive income (loss).” Gains and losses resulting from foreign currency transactions are included in the statements of operations and comprehensive income (loss) as other comprehensive income (loss). There have been no significant fluctuations in the exchange rate for the conversion of Australian dollars to USD after the balance sheet date.
Other Comprehensive Income (Loss) for all periods presented includes only foreign currency translation gains (losses).
Assets and liabilities denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing at the consolidated balance sheet date with any transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency included in the consolidated results of operations as incurred.
As of December 31, 2018 and June 30, 2018, the exchange rates used to translate amounts in Australian dollars into USD for the purposes of preparing the consolidated financial statements were as follows:
| December 31, 2018 | June 30, 2018 | |||||||
| Exchange rate on balance sheet dates | ||||||||
| USD : AUD exchange rate | 0.7406 | 0.7399 | ||||||
| Average exchange rate for the period | ||||||||
| USD : AUD exchange rate | 0.7248 | 0.7753 | ||||||
The exchange rates used to translate amounts in AUD into USD for the period ended December 31, 2017 are: 0.7815 as of the balance sheet date and 0.7795 average exchange rate for that period.
| F-7 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
Change in Accumulated Other Comprehensive Income (Loss) by component during the six months ended December 31, 2018 was as follows:
| Foreign
Currency Items: | ||||
| Beginning balance, June 30, 2018 | $ | 357,929 | ||
| Foreign currency translation gain | 634,495 | |||
| Ending balance, December 31, 2018 | $ | 992,424 | ||
Fair Value of Financial Instruments and Fair Value Measurements
The Company measures its financial assets and liabilities in accordance with US GAAP. For certain financial instruments, including cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities, the carrying amounts approximate fair value due to their short maturities. Amounts recorded for notes payable, net of discount, and loans payable also approximate fair value because current interest rates available for debt with similar terms and maturities are substantially the same.
The Company follows accounting guidance for financial assets and liabilities. This standard defines fair value, provides guidance for measuring fair value and requires certain disclosures. This standard does not require any new fair value measurements, but rather applies to all other accounting pronouncements that require or permit fair value measurements. This guidance does not apply to measurements related to share-based payments. This guidance discusses valuation techniques, such as the market approach (comparable market prices), the income approach (present value of future income or cash flow), and the cost approach (cost to replace the service capacity of an asset or replacement cost).
The guidance utilizes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The following is a brief description of those three levels:
Level 1: Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2: Inputs, other than quoted prices that are observable, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active.
Level 3: Unobservable inputs in which little or no market data exists, therefore developed using estimates and assumptions developed by us, which reflect those that a market participant would use.
Also see Note 10 - Derivative Financial Instruments and Fair Value Measurements.
Cash and Cash Equivalents
Cash and cash equivalents include cash on hand and at banks, short-term deposits with an original maturity of three months or less with financial institutions, and bank overdrafts. Bank overdrafts are reflected as a current liability on the balance sheets. There were no cash equivalents as of December 31, 2018 or June 30, 2018.
Patents
Patents are stated at cost and reclassified to intangible assets and amortized on a straight-line basis over the estimated future periods if and once the patent has been granted by a regulatory agency. However, the Company will expense any product costs as long as we are in the startup stage. Accordingly, as the Company’s products were and are not currently approved for market, all patent costs incurred from 2013 through 2018 were expensed immediately. This practice of expensing patent costs immediately ends when a product receives market authorization from a government regulatory agency.
| F-8 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
Impairment of Long-Lived Assets
In accordance with ASC 360-10, “Long-lived assets,” which include property and equipment and intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of long-lived assets to be held and used is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the assets. Fair value is generally determined using the asset’s expected future discounted cash flows or market value, if readily determinable.
Australian Goods and Services Tax (“GST”)
Revenues, expenses and balance sheet items are recognized net of the amount of GST, except payable and receivable balances which are shown inclusive of GST. The GST incurred is payable on revenues to, and recoverable on purchases from, the Australian Taxation Office.
Cash flows are presented in the statements of cash flow on a gross basis, except for the GST component of investing and financing activities, which are disclosed as operating cash flows.
As of December 31, 2018 and June 30, 2018, the Company was owed $8,639 and $6,257, respectively, from the Australian Taxation Office. These amounts were fully collected subsequent to the balance sheet reporting dates.
Derivative Instruments
ASC Topic 815, Derivatives and Hedging (“ASC Topic 815”), establishes accounting and reporting standards for derivative instruments and for hedging activities by requiring that all derivatives be recognized in the balance sheet and measured at fair value. Gains or losses resulting from changes in the fair value of derivatives are recognized in earnings. On the date of conversion or payoff of debt, the Company records the fair value of the conversion shares, removes the fair value of the related derivative liability, removes any discounts and records a net gain or loss on debt extinguishment.
Convertible Notes With Variable Conversion Options
The Company has entered into convertible notes, some of which contain variable conversion options, whereby the outstanding principal and accrued interest may be converted, by the holder, into common shares at a fixed discount to the price of the common stock at the time of conversion. The Company treats these convertible notes as stock settled debt under ASC 480, “Distinguishing Liabilities from Equity” and measures the fair value of the notes at the time of issuance, which is the result of the share price discount at the time of conversion and records the put premium as accretion to interest expense to the date of first conversion.
Income Taxes
The Company is governed by Australia and United States income tax laws, which are administered by the Australian Taxation Office and the United States Internal Revenue Service, respectively. The Company follows ASC 740 “Accounting for Income Taxes,” when accounting for income taxes, which requires an asset and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed annually for temporary differences between the financial statements and tax bases of assets and liabilities that will result in taxable or deductible amounts in the future based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Income tax expense is the tax payable or refundable for the period plus or minus the change during the period in deferred tax assets and liabilities.
The Company follows ASC 740, Sections 25 through 60, “Accounting for Uncertainty in Income Taxes.” These sections provide detailed guidance for the financial statement recognition, measurement and disclosure of uncertain tax positions recognized in the financial statements. Tax positions must meet a “more-likely-than-not” recognition threshold at the effective date to be recognized upon the adoption of ASC 740 and in subsequent periods.
| F-9 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
On December 22, 2017, the passage of legislation commonly referred to as the Tax Cuts and Jobs Act (“TCJA”) was enacted and significantly revised the U.S. income tax law. The TCJA includes changes, which reduce the corporate income tax rate from 34% to 21% for fiscal years beginning after December 31, 2017. On December 22, 2017, the SEC Staff Accounting Bulletin No. 118 (“SAB 118”) was issued, which allows a company to recognize provisional tax amounts when it does not have the necessary information available, prepared or analyzed, including computations, in reasonable detail to complete its accounting for the change in tax law. SAB 118 provides for a measurement of up to one year from the date of enactment.
Research and Development Costs and Tax Credits
In accordance with ASC 730-10, “Research and Development-Overall,” research and development costs are expensed when incurred. Total research and development costs for the three months ended December 31, 2018 and 2017 were $94,777 and $1,034,729, respectively. Total research and development costs for the six months ended December 31, 2018 and 2017 were $150,970 and $1,598,468, respectively.
The Company may apply for research and development tax concessions with the Australian Taxation Office on an annual basis. Although the amount is possible to estimate at year end, the Australian Taxation Office may reject or materially alter the claim amount. Accordingly, the Company does not recognize the benefit of the claim amount until cash receipt since collectability is not certain until such time. The tax concession is a refundable credit. If the Company has net income, then the Company can receive the credit which reduces its income tax liability. If the Company has net losses, then the Company may still receive a cash payment for the credit, however, the Company’s net operating loss carryforwards are reduced by the gross equivalent loss that would produce the credit amount when the income tax rate is applied to that gross amount. The concession is recognized as an income tax benefit, in operations, upon receipt.
During each of the six months ended December 31, 2018 and 2017, the Company applied for, and received from the Australian Taxation Office, a research and development tax credit in the amount of $116,970 and $180,278 respectively, which is reflected as a tax benefit in the accompanying condensed consolidated statements of operations and comprehensive income (loss).
Stock Based Compensation
The Company records stock-based compensation in accordance with ASC 718, “Stock Compensation” and the SEC Staff Accounting Bulletin No. 107 Share Based Payment was issued in March 2005 regarding its interpretation of ASC 718. ASC 718 requires the fair value of all stock-based employee compensation awarded to employees to be recorded as an expense over the related requisite service period. The Company values employee and non-employee stock-based compensation at fair value using the Black-Scholes Option Pricing Model.
The Company accounts for non-employee share-based awards in accordance with the measurement and recognition criteria of ASC 505-50 “Equity-Based Payments to Non-Employees.”
Basic and Diluted Net Loss Per Common Share
Basic net loss per share is computed by dividing the net loss by the weighted average number of common shares outstanding during the period. Diluted net loss per common share is computed by dividing the net loss by the weighted average number of common shares outstanding for the period and, if dilutive, potential common shares outstanding during the period. Potentially dilutive securities consist of the incremental common shares issuable upon exercise of common stock equivalents such as stock options, warrants and convertible debt instruments. Potentially dilutive securities are excluded from the computation if their effect is anti-dilutive. As a result, the basic and diluted per share amounts for all periods presented are identical. As of December 31, 2018, there were 29,517 warrants outstanding, 572,000 stock options and 14 convertible notes payable, which notes are convertible into 215,153,929 shares of the Company’s common stock. Each holder of the notes has agreed to a 4.99% beneficial ownership conversion limitation (subject to certain noteholders’ ability to increase such limitation to 9.99% upon 60 days’ notice to the Company), and each note may not be converted during the first six-month period from the date of issuance. Such securities are considered dilutive securities which were excluded from the computation since the effect is anti-dilutive.
| F-10 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
Recently Adopted Accounting Pronouncements
Certain FASB Accounting Standard Updates (“ASU”) that are not effective until after December 31, 2018 are not expected to have a significant effect on the Company’s consolidated financial position or results of operations. The Company is evaluating or has implemented the following at December 31, 2018:
ASU 2018-07 - In June 2018, the Financial Accounting Standards Board (the “FASB”) issued Accounting Standards Update (“ASU”) 2018-07, Compensation – Stock Compensation (Topic 718). This update is intended to reduce cost and complexity and to improve financial reporting for share-based payments issued to non-employees (for example, service providers, external legal counsel, suppliers, etc.). The ASU expands the scope of Topic 718, Compensation—Stock Compensation, which currently only includes share-based payments issued to employees, to also include share-based payments issued to non-employees for goods and services. Consequently, the accounting for share-based payments to non-employees and employees will be substantially aligned. This standard will be effective for financial statements issued by public companies for the annual and interim periods beginning after December 15, 2018. Early adoption of the standard is permitted. The standard will be applied in a retrospective approach for each period presented. Management currently does not plan to early adopt this guidance and believes the impact of this guidance will not be material to the Company’s consolidated financial statements upon implementation on January 1, 2019.
ASU 2016-02 - In February 2016, the FASB issued ASU No. 2016-02: “Leases (Topic 842)” whereby lessees will need to recognize almost all leases on their balance sheet as a right of use asset and a lease liability. This guidance is effective for interim and annual reporting periods beginning after December 15, 2018. The Company does not anticipate this ASU to have a material impact on its consolidated financial statements when implemented on January 1, 2019.
ASU 2014-09 - In May 2014, the FASB issued ASU No. 2014-09: “Revenue from Contracts with Customers (Topic 606)” which requires that an entity recognize revenue to depict the transfer of promised goods and services to customers in an amount that reflects the consideration to which the Company expects to be entitled in exchange for those goods or services. Since the issuance of the original standard, the FASB has issued several updates to the standard which (i) clarify the application of the principal versus agent guidance; (ii) clarify the guidance relating to performance obligations and licensing; (iii) clarify assessment of the collectability criterion, presentation of sales taxes, measurement date for non-cash consideration and completed contracts at transaction; and (iv) clarify narrow aspects of ASC 606 or corrects unintended application of the guidance. The new revenue recognition standard, amended by the updates, becomes effective in the first quarter of fiscal 2019 and is to be applied retrospectively using one of two prescribed methods. The Company adopted this new standard effective July 1, 2018.
NOTE 2 – GOING CONCERN
The accompanying condensed consolidated financial statements have been prepared in conformity with US GAAP, which contemplate continuation of the Company as a going concern. For the six months ended December 31, 2018, the Company had no revenues, had a net loss of $3,574,974 and had net cash used in operations of $1,056,786. Additionally, as of December 31, 2018, the Company had a working capital deficit, stockholders’ deficit and accumulated deficit of $4,228,578, $3,904,036 and $48,857,652, respectively. It is management’s opinion that these conditions raise substantial doubt about the Company’s ability to continue as a going concern for a period of twelve months from the date of this filing.
The condensed consolidated financial statements do not include any adjustments to reflect the possible future effect on the recoverability and classification of assets or the amounts and classifications of liabilities that may result from the outcome of this uncertainty.
| F-11 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
Successful completion of the Company’s development program and, ultimately, the attainment of profitable operations are dependent upon future events, including obtaining adequate financing to fulfill its development activities, acceptance of the Company’s patent applications, obtaining additional sources of suitable and adequate financing and ultimately achieving a level of sales adequate to support the Company’s cost structure and business plan. The Company’s ability to continue as a going concern is also dependent on its ability to further develop and execute on its business plan. However, there can be no assurances that any or all of these endeavors will be successful.
NOTE 3 – DUE TO FORMER DIRECTOR - RELATED PARTY
Due to director - related party represents unsecured advances made primarily by a former director for operating expenses on behalf of the Company such as intellectual property and formation expenses. The expenses were paid for on behalf of the Company and are due upon demand. The Company is currently not being charged interest under these advances. The total amount owed the former director at December 31, 2018 and June 30, 2018 is $31,329 and $32,898, respectively. The Company plans to repay the notes as its cash resources allow.
NOTE 4 – LOANS AND NOTES PAYABLE
Loans from Directors and Officer - Related Parties
Loans from the Company’s directors and officer at December 31, 2018 and June 30, 2018 were $52,140 and $54,753, respectively. The loans bear no interest and are all payable on demand. The Company did not repay any amount on these loans during the six months ended December 31, 2018.
NOTE 5 – CONVERTIBLE NOTES
The Company’s convertible notes outstanding at December 31, 2018 were as follows:
| Convertible notes and debenture | $ | 1,672,385 | ||
| Unamortized discounts | (79,469 | ) | ||
| Accrued interest | 83,706 | |||
| Premiums | 1,122,002 | |||
| Convertible notes, net | $ | 2,789,624 |
Eagle Equities Financing Agreements
December 12, 2016 Securities Purchase Agreement
On December 12, 2016, the Company entered into a Securities Purchase Agreement, with Eagle Equities, pursuant to which Eagle Equities purchased two 8% convertible redeemable junior subordinated promissory notes, each in the principal amount of $100,000. The first note (the “December 12 Note”) was funded with cash and the second note (the “December 12 Eagle Back-End Note”) was initially paid for by an offsetting promissory note issued by Eagle Equities to the Company (the “December 12 Note Receivable”). The terms of the December 12 Eagle Back-End Note require cash funding prior to any conversion thereunder. The December 12 Note Receivable is due December 12, 2017, unless certain conditions are not met, in which case both the December 12 Eagle Back-End Note and the December 12 Note Receivable may both be cancelled. Both the December 12 Note and the December 12 Eagle Back-End Note have a maturity date one year from the date of issuance upon which any outstanding principal and interest is due and payable. The outstanding principal amounts plus accrued interest under both the December 12 Note and the December 12 Eagle Back-End Note are convertible into the Company’s common stock at a conversion price equal to 60% of the lowest closing bid price of the common stock for the ten trading days prior to the conversion, subject to adjustment in certain events. On April 11, 2017, the Company received payment of the December 12 Note Receivable in the amount of $100,000 that offset the December Eagle Back-End Note. Proceeds from the Note Receivable of $5,000 were paid directly to legal fees resulting in net cash proceeds of $95,000 received by the Company. As a result, the December 12 Eagle Back-End Note is now convertible. The December 12 Note and the December 12 Eagle Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company recorded a put premium of $66,667 as each of the notes were funded. As of June 30, 2018, the outstanding principal under the December 12 Note along with $8,296 of accrued interest was fully converted into shares of the Company’s common stock. As of December 31, 2018, the outstanding balance of $100,000 under the December 12 Eagle Back-End Note along with $13,144 of accrued interest was fully converted (see Note 6 – Stockholders’ Deficit) resulting in full repayment of the note and a full reduction of the put premium.
| F-12 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
January 27, 2017 Securities Purchase Agreement
On January 27, 2017, the Company entered into a Securities Purchase Agreement with Eagle Equities, LLC, pursuant to which Eagle Equities purchased two 8% convertible redeemable junior subordinated promissory notes, each in the principal amount of $230,000. The first note (the “January 2017 Eagle Note”) was funded with cash and the second note (the “January 2017 Eagle Back-End Note”) was initially paid for by an offsetting promissory note issued by Eagle Equities to the Company (the “January 2017 Eagle Note Receivable”). The terms of the January 2017 Eagle Back-End Note require cash funding prior to any conversion thereunder. The January 2017 Eagle Note Receivable is due September 27, 2017, unless certain conditions are not met, in which case both the January 2017 Eagle Back-End Note and the January 2017 Eagle Note Receivable may both be cancelled. Both the January 2017 Eagle Note and the January 2017 Eagle Back-End Note have a maturity date one year from the date of issuance upon which any outstanding principal and interest is due and payable. The outstanding principal amounts plus accrued interest under both the January 2017 Eagle Note and the January 2017 Eagle Back-End Note are convertible into common stock of the Company at a conversion price equal to 60% of the lowest closing bid price of the common stock for the ten trading days prior to the conversion, subject to adjustment in certain events. On May 4, 2017, the Company received a partial payment of the January 2017 Note Receivable in the amount of $40,000 and on June 3, 2017 the balance of $190,000 was funded, of which $11,250 was paid directly to legal fees. As a result, the January 2017 Eagle Back-End Note is now convertible. The January 2017 Eagle Note and the January 2017 Eagle Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company is recording a put premium of $153,333 as each of the notes were funded. As of June 30, 2018, the outstanding principal under the January 2017 Eagle Note along with $14,988 of accrued interest was fully converted. As of December 31, 2018, the outstanding balance of $230,000 under the January 2017 Eagle Back-End Note along with $33,356 of accrued interest was fully converted (see Note 6 – Stockholders’ Deficit) resulting in full repayment of the note and a full reduction of the put premium.
March 1, 2017 Securities Purchase Agreement
On March 1, 2017, the Company entered into a Securities Purchase Agreement with Eagle Equities, pursuant to which Eagle Equities purchased two 8% convertible redeemable junior subordinated promissory notes, each in the principal amount of $220,500. The first note (the “March 2017 Eagle Note”) was funded with cash and the second note (the “March 2017 Eagle Back-End Note”) was initially paid for by an offsetting promissory note issued by Eagle Equities to the Company (the “March 2017 Eagle Note Receivable”). The terms of the March 2017 Eagle Back-End Note require cash funding prior to any conversion thereunder. Both the March 2017 Eagle Note and the March 2017 Eagle Back-End Note had a maturity date of March 1, 2018, upon which any outstanding principal and interest was due and payable. The outstanding principal amounts plus accrued interest under both the March 2017 Eagle Note and the March 2017 Eagle Back-End Note are convertible into shares of common stock, of the Company at a conversion price equal to 60% of the lowest closing bid price of the common stock for the ten trading days prior to the conversion, subject to adjustment in certain events. On July 5, 2017, the Company received payment of the March 2017 Eagle Note Receivable in the amount of $220,500 that offset the March 2017 Eagle Back-End Note. Proceeds from the March 2017 Eagle Note Receivable of $10,500 were paid directly to legal fees resulting in net cash proceeds of $210,000 received by the Company. As a result, the March 2017 Eagle Back-End Note is now convertible. The March 2017 Eagle Note and the March 2017 Eagle Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company recorded a put premium of $147,000 as each of the notes were funded. As of June 30, 2018, the outstanding principal balance under the March 2017 Eagle Note along with $20,061 of accrued interest was fully converted. As of December 31, 2018, the outstanding balance of $220,500 under the March 2017 Back-End Note along with $18,625 of accrued interest was fully converted (see Note 6 – Stockholders’ Deficit) resulting in a full reduction of the put premium.
| F-13 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
August 9, 2017 Securities Purchase Agreement
On August 9, 2017, the Company entered into a Securities Purchase Agreement dated as of August 8, 2017, with Eagle Equities, pursuant to which Eagle Equities purchased two 8% convertible redeemable junior subordinated promissory notes, each in the principal amount of $200,000. The first note (the “August 2017 Eagle Note”) was funded with cash and the second note (the “August 2017 Eagle Back-End Note”) was initially paid for by an offsetting promissory note issued by Eagle Equities to the Company (the “August 2017 Eagle Note Receivable”). The terms of the August 2017 Eagle Back-End Note require cash funding prior to any conversion thereunder. The August 2017 Eagle Note Receivable is due August 8, 2018, unless certain conditions are not met, in which case both the August 2017 Eagle Back-End Note and the August 2017 Eagle Note Receivable may both be cancelled. Both the August 2017 Eagle Note and the August 2017 Eagle Back-End Note have a maturity date one year from the date of issuance upon which any outstanding principal and interest is due and payable. The outstanding principal amounts plus accrued interest under both the August 2017 Eagle Note and the August 2017 Eagle Back-End Note are convertible into common stock of the Company at a conversion price equal to 60% of the lowest closing bid price of the common stock for the ten trading days prior to the conversion, subject to adjustment in certain events. On September 14, 2017, the Company received payment of the August 2017 Eagle Note Receivable in the amount of $200,000 that offset the August 2017 Eagle Back-End Note. Proceeds from the August 2017 Eagle Note Receivable of $10,000 were paid directly to legal fees resulting in net cash proceeds of $190,000 received by the Company. As a result, the August 2017 Eagle Back-End Note is now convertible. The August 2017 Eagle Note and the August 2017 Eagle Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company recorded a put premium of $133,333 as each of the notes were funded. As of June 30, 2018 $120,000 of principal under the August 2017 Eagle Note along with $5,273 in interest was converted. As of December 31, 2018, the remaining outstanding balance of $80,000 under the August 2017 Eagle Note along with $6,850 of accrued interest was fully converted (see Note 6 – Stockholders’ Deficit) resulting in full repayment of the note and a full reduction of the put premium. As of December 31, 2018, The Company has recorded $5,249 of accrued interest for the August 2017 Eagle Back-End Note and total principal outstanding as of December 31, 2018 under the August 2017 Eagle Back-End Note was $65,000 as $135,000 was converted during the six months ended December 31, 2018 (see Note 6 – Stockholders’ Deficit). The Company is currently in discussions with Eagle Equities to extend the maturity date.
Upon an event of default, principal and accrued interest will become immediately due and payable under the notes. Additionally, upon an event of default, both notes will accrue interest at a default interest rate of 24% per annum or the highest rate of interest permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
October 25, 2017 Securities Purchase Agreement
On November 3, 2017, the Company entered into a Securities Purchase Agreement dated as of October 25, 2017, with Eagle Equities, pursuant to which Eagle Equities purchased two 8% convertible redeemable junior subordinated promissory notes, each in the principal amount of $200,000. The first note (the “October 2017 Eagle Note”) was funded with cash and the second note (the “October 2017 Eagle Back-End Note”) was initially paid for by an offsetting promissory note issued by Eagle Equities to the Company (the “October 2017 Eagle Note Receivable”). The terms of the October 2017 Eagle Back-End Note require cash funding prior to any conversion thereunder. The October 2017 Eagle Note Receivable is due June 25, 2018, unless certain conditions are not met, in which case both the October 2017 Eagle Back-End Note and the October 2017 Eagle Note Receivable may both be cancelled. Both the October 2017 Eagle Note and the October 2017 Eagle Back-End Note have a maturity date one year from the date of issuance upon which any outstanding principal and interest is due and payable. The amounts cash funded plus accrued interest under both the October 2017 Eagle Note and the October 2017 Eagle Back-End Note are convertible into common stock, par value $0.001 of the Company at a conversion price equal to 60% of the lowest closing bid price of the common stock for the ten trading days prior to the conversion, subject to adjustment in certain events. On December 6, 2017, the Company received payment of the October 2017 Eagle Note Receivable in the amount of $200,000 that offset the October 2017 Eagle Back-End Note. Proceeds from the October 2017 Eagle Note Receivable of $10,000 were paid directly to legal fees resulting in net cash proceeds of $190,000 received by the Company. As a result, the October 2017 Eagle Back-End Note is now convertible. The October 2017 Eagle Note and the October 2017 Eagle Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company recorded a put premium of $133,333 as each of the notes were funded. As of December 31, 2018, the outstanding principal balance under the October 2017 Eagle Note along with $14,261 of accrued interest was fully converted (see Note 6 – Stockholders’ Deficit) resulting in full repayment of the note and a full reduction of the put premium. As of December 31, 2018, the outstanding principal balance under the October 2017 Eagle Back-End Note along with $13,417 of accrued interest was fully converted (see Note 6 – Stockholders’ Deficit) resulting in full repayment of the note and a full reduction of the put premium.
| F-14 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
December 29, 2017 Securities Purchase Agreement
The Company entered into an executory contract on December 29, 2017, whereby the Company entered into a securities purchase agreement with Eagle Equities, pursuant to which Eagle Equities purchased a convertible promissory note (the “December 2017 Eagle Note”) from the Company in the aggregate principal amount of $532,435, with principal and the interest thereon convertible into shares of the Company’s common stock at the option of Eagle Equities at any time. The transactions closed on January 2, 2018.
The December 2017 Eagle Note contains an original issue discount of $25,354 such that the purchase price was $507,081. The maturity date of the December 2017 Eagle Note was December 29, 2018. The December 2017 Eagle Note bears interest at a rate of 8% per annum, which interest shall be paid by the Company to Eagle Equities in shares of the Company’s common stock upon receipt of a notice of conversion by the Company from Eagle Equities at any time. The Company has recorded $42,478 of accrued interest as of December 31, 2018 for the December 2017 Eagle Note and total principal outstanding as of December 31, 2018 under the December 2017 Eagle Note was $532,435. The Company is currently in discussions with Eagle Equities to extend the maturity date.
Eagle Equities has the option to convert all or any amount of the principal face amount of the December 2017 Eagle Note, at any time, for shares of the Company’s common stock at a price equal to 60% of the lowest closing bid price of the Company’s common stock as reported on the OTC Markets quotation system for the ten prior trading days, including the day upon which the Company receives a notice of conversion from Eagle Equities. The note is treated as stock settled debt under ASC 480 and accordingly the Company recorded a $354,956 put premium.
June 14, 2018 Securities Purchase Agreement
Effective June 14, 2018, the Company entered into a securities purchase agreement with Eagle Equities, pursuant to which Eagle Equities purchased a convertible promissory note (the “June 2018 Eagle Note”) from the Company in the aggregate principal amount of $105,000, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Eagle Equities any time after the six-month anniversary of the June 2018 Eagle Note. The transactions contemplated by the Purchase Agreement closed on June 19, 2018. Pursuant to the terms of the Purchase Agreement, Eagle Equities deducted $5,000 from the principal payment due under the June 2018 Eagle Note, at the time of closing, to be applied to its legal expenses.
The maturity date of the June 2018 Eagle Note is June 14, 2019. The June 2018 Eagle Note bears interest at a rate of 8% per annum, which interest shall be paid by the Company to Eagle Equities in shares of the Company’s common stock upon receipt of a notice of conversion by the Company from Eagle Equities at any time after the six-month anniversary of the June 2018 Eagle Note.
Additionally, Eagle Equities has the option to convert all or any amount of the principal face amount of the June 2018 Eagle Note, at any time, for shares of the Company’s common stock at a price equal to 60% of the lowest closing bid price of the Company’s common stock as reported on the OTC quotation system for the ten prior trading days, including the day upon which the Company receives a notice of conversion from Eagle Equities. However, in the event that the Company’s common stock is restricted by the Depository Trust Company (“DTC”) for any reason, the Conversion Price shall be lowered to 50% of the lowest closing bid price for the duration of such restriction. If the Company fails to maintain a reserve of shares of its common stock at least four times the number of shares issuable upon conversion of the Note for at least 60 days after the issuance of the Note, the conversion discount shall be increased by 10%. Notwithstanding the foregoing, Eagle Equities shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Eagle Equities and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock. The June 2018 Eagle Note is treated as stock settled debt under ASC 480 and accordingly, the Company recorded a $70,000 put premium. The Company has recorded $4,603 of accrued interest and the total principal outstanding under the June 2018 Eagle Note was $105,000 as of December 31, 2018.
The June 2018 Eagle Note may be prepaid by the Company with certain penalties until December 11, 2018. No payment has been made through December 31, 2018.
| F-15 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
Upon an event of default, principal and accrued interest will become immediately payable under the note. Interest on the outstanding principal shall accrue at a default interest rate of 24% per annum or at the highest rate permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
July 13, 2018 Securities Purchase Agreement
Effective July 13, 2018, the Company entered into a securities purchase agreement with Eagle Equities, pursuant to which Eagle Equities purchased a convertible promissory note (the “July 2018 Note”) from the Company in the aggregate principal amount of $75,000, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Eagle Equities any time after the six month anniversary of the July 2018 Eagle Note. The transaction closed on July 16, 2018 and on July 19, 2018 the Company received proceeds of $71,250 as $3,750 was paid directly to legal fees.
The maturity date of the July 2018 Eagle Note is July 13, 2019. The July 2018 Eagle Note bears interest at a rate of 8% per annum, which interest shall be paid by the Company to Eagle Equities in shares of the Company’s common stock upon receipt of a notice of conversion by the Company from Eagle Equities at any time after the six-month anniversary of the Note.
Additionally, Eagle Equities has the option to convert all or any amount of the principal face amount of the July 2018 Eagle Note, at any time, for shares of the Company’s common stock at a price equal to 60% of the lowest closing bid price of the Company’s common stock for the ten prior trading days, including the day upon which the Company receives a notice of conversion, subject to adjustment in certain events. Eagle Equities shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Eagle Equities and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock. The July 2018 Eagle Note is treated as stock settled debt under ASC 480 and accordingly, the Company recorded a $50,000 put premium. The Company has recorded $2,811 of accrued interest and the total principal outstanding under the July 2018 Eagle Note was $75,000 as of December 31, 2018. The July 2018 Eagle Note may be prepaid by the Company with certain penalties until January 9, 2019.
Upon an event of default, principal and accrued interest will become immediately due and payable under the notes. Additionally, upon an event of default, both notes will accrue interest at a default interest rate of 24% per annum or the highest rate of interest permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
August 29, 2018 Securities Purchase Agreement
Effective August 29, 2018, the Company entered into a securities purchase agreement with Eagle Equities, pursuant to which Eagle Equities purchased a convertible promissory note (the “August 2018 Eagle Note”) from the Company in the aggregate principal amount of $105,000, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Eagle Equities any time after the six-month anniversary of the August 2018 Eagle Note. The transactions contemplated by the agreement closed on August 30, 2018.
The maturity date of the August 29, 2018 Eagle Note is August 2019. The August 2018 Eagle Note bears interest at a rate of 8% per annum, which interest shall be paid by the Company to Eagle Equities in shares of the Company’s common stock upon receipt of a notice of conversion by the Company from Eagle Equities at any time after the six-month anniversary of the August 2018 Eagle Note.
Additionally, Eagle Equities has the option to convert all or any amount of the principal face amount of the August 2018 Eagle Note, at any time, into shares of the Company’s common stock at a price equal to 60% of the lowest closing bid price (the “Closing Bid Price”) of the Company’s common stock as reported on the OTC Markets quotation system for the ten prior trading days, including the day upon which the Company receives a notice of conversion from Eagle Equities (the “Conversion Price”). However, in the event that the Company’s common stock is restricted by the DTC for any reason, the Conversion Price shall be lowered to 50% of the lowest Closing Bid Price for the duration of such restriction. If the Company fails to maintain a reserve of shares of its common stock at least four times the number of shares issuable upon conversion of the August 2018 Eagle Note for at least 60 days after the issuance of the August 28, 2018 Eagle Note, the conversion discount shall be increased by 10%. Notwithstanding the foregoing, Eagle Equities shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Eagle Equities and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock. The August 2018 Eagle Note is treated as stock settled debt under ASC 480 and accordingly, the Company recorded a $70,000 put premium. The Company has recorded $2,877 of accrued interest and the total principal outstanding under the August 2018 Eagle Note was $105,000 as of December 31, 2018. The August 2018 Eagle Note may be prepaid by the Company until February 25, 2019.
| F-16 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
Upon an event of default, interest on the outstanding principal shall accrue at a default interest rate of 24% per annum or at the highest rate permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
October 2, 2018 Securities Purchase Agreement
Effective October 2, 2018, the Company entered into a securities purchase agreement with Eagle Equities, pursuant to which Eagle Equities purchased a convertible promissory note (the “October 2018 Eagle Note”) from the Company in the aggregate principal amount of $210,000, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Eagle Equities any time after the six month anniversary of the October 2018 Eagle Note. The transactions contemplated by the purchase agreement closed on October 3, 2018. Pursuant to the terms of the purchase agreement, Eagle Equities deducted $10,000 from the principal payment due under the October 2018 Eagle Note, at the time of closing, to be applied to its legal expenses.
The maturity date of the October 2018 Eagle Note is October 2, 2019. The October 2018 Eagle Note shall bear interest at a rate of 8% per annum, which interest shall be paid by the Company to Eagle Equities in shares of common stock upon receipt of a notice of conversion by the Company from Eagle Equities at any time after the six month anniversary of the October 2018 Eagle Note.
Additionally, Eagle Equities has the option to convert all or any amount of the principal amount of the October 2018 Eagle Note, at any time, for shares of the Company’s common stock at a price equal to 60% of the lowest closing bid price of the Company’s common stock for the ten prior trading days, including the day upon which the Company receives a notice of conversion, subject to adjustment in certain events. Eagle Equities shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Eagle Equities and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock. The October 2, 2018 Eagle Note is treated as stock settled debt under ASC 480 and accordingly, the Company recorded a $140,000 put premium. The Company has recorded $4,142 of accrued interest and the total principal outstanding under the October 2018 Eagle Note was $210,000 as of December 31, 2018. The October 2018 Eagle Note may be prepaid with certain penalties until March 31, 2019.
Upon an event of default, principal and accrued interest will become immediately due and payable under the notes. Additionally, upon an event of default, both notes will accrue interest at a default interest rate of 24% per annum or the highest rate of interest permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
November 30, 2018 Securities Purchase Agreement
Effective November 30, 2018, the Company entered into a securities purchase agreement with Eagle Equities, pursuant to which Eagle Equities purchased a convertible promissory note (the “November 2018 Eagle Note”) from the Company in the aggregate principal amount of $105,000, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Eagle Equities any time after the six month anniversary of the November 2018 Eagle Note. The transactions contemplated by the purchase agreement closed on December 3, 2018. Pursuant to the terms of the purchase agreement, Eagle Equities deducted $5,000 from the principal payment due under the November 2018 Eagle Note, at the time of closing, to be applied to its legal expenses.
The maturity date of the November 2018 Eagle Note is November 30, 2019. The November 2018 Eagle Note shall bear interest at a rate of 8% per annum, which interest shall be paid by the Company to Eagle Equities in shares of common stock upon receipt of a notice of conversion by the Company from Eagle Equities at any time after the six month anniversary of the November 2018 Eagle Note.
| F-17 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
Additionally, Eagle Equities has the option to convert all or any amount of the principal amount of the November 2018 Eagle Note, at any time, for shares of the Company’s common stock at a price equal to 61% of the lowest closing bid price (the “Closing Bid Price”) of the Company’s common stock as reported on the OTC Markets Group, Inc. quotation system for the ten prior trading days, including the day upon which the Company receives a notice of conversion from Eagle Equities (the “Conversion Price”). However, in the event that the Company’s common stock is restricted by the Depository Trust Company for any reason, the Conversion Price shall be lowered to 51% of the lowest Closing Bid Price for the duration of such restriction. If the Company fails to maintain a reserve of shares of its common stock at least two and a half times the number of shares issuable upon conversion of the November 2018 Eagle Note for at least 60 days after the issuance of the November 2018 Eagle Note, the conversion discount shall be increased by 10%. Notwithstanding the foregoing, Eagle Equities shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Eagle Equities and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock. The November 2018 Eagle Note is treated as stock settled debt under ASC 480 and accordingly, the Company recorded a $67,131 put premium. The Company has recorded $713 of accrued interest and the total principal outstanding under the November 2018 Eagle Note was $105,000 as of December 31, 2018. The November 2018 Eagle Note may be prepaid with certain penalties until May 29, 2019.
Upon an event of default, principal and accrued interest will become immediately due and payable under the notes. Additionally, upon an event of default, both notes will accrue interest at a default interest rate of 24% per annum or the highest rate of interest permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
December 24, 2018 Securities Purchase Agreement
Effective December 24, 2018, the Company entered into a securities purchase agreement with Eagle Equities, pursuant to which Eagle Equities purchased a convertible promissory note (the “December 2018 Eagle Note”) from the Company in the aggregate principal amount of $126,000, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Eagle Equities any time after the six month anniversary of the December 2018 Eagle Note. The transactions contemplated by the purchase agreement closed on December 24, 2018. Pursuant to the terms of the purchase agreement, Eagle Equities deducted $6,000 from the principal payment due under the December 2018 Eagle Note, at the time of closing, to be applied to its legal expenses. The Company used the net proceeds from the December 2018 Eagle Note to repay an outstanding convertible promissory note before such note became convertible.
The maturity date of the December 2018 Eagle Note is December 24, 2019. The December 2018 Eagle Note shall bear interest at a rate of 8% per annum, which interest shall be paid by the Company to Eagle Equities in shares of common stock upon receipt of a notice of conversion by the Company from Eagle Equities at any time after the six-month anniversary of the December 2018 Eagle Note.
Additionally, Eagle Equities has the option to convert all or any amount of the principal amount of the December 2018 Eagle Note, at any time, for shares of the Company’s common stock at a price equal to 61% of the lowest closing bid price (the “Closing Bid Price”) of the Company’s common stock as reported on the OTC Markets Group, Inc. quotation system for the ten prior trading days, including the day upon which the Company receives a notice of conversion from Eagle Equities (the “Conversion Price”). However, in the event that the Company’s common stock is restricted by the Depository Trust Company for any reason, the Conversion Price shall be lowered to 51% of the lowest Closing Bid Price for the duration of such restriction. If the Company fails to maintain a reserve of shares of its common stock at least two and a half times the number of shares issuable upon conversion of the December 2018 Eagle Note for at least 60 days after the issuance of the December 2018 Eagle Note, the conversion discount shall be increased by 10%. Notwithstanding the foregoing, Eagle Equities shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Eagle Equities and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock. The December 2018 Eagle Note is treated as stock settled debt under ASC 480 and accordingly, the Company recorded an $80,557 put premium. The Company has recorded $221 of accrued interest and the total principal outstanding under the November 2018 Eagle Note was $126,000 as of December 31, 2018. The December 2018 Eagle Note may be prepaid with certain penalties until June 22, 2019.
| F-18 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
Upon an event of default, principal and accrued interest will become immediately due and payable under the notes. Additionally, upon an event of default, both notes will accrue interest at a default interest rate of 24% per annum or the highest rate of interest permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
The total principal amount outstanding under the above Eagle Equities financing agreements, specifically the August 9, 2017, the December 29, 2017, the June 14, 2018, the July 13, 2018, the August 29, 2018, the October 2, 2018, the November 30, 2018 and the December 24, 2018 agreements was $1,323,435 as of December 31, 2018 and accrued interest totaled $63,094.
GS Capital Financing Agreements
July 24, 2017 Securities Purchase Agreement
On July 24, 2017, the Company entered into a Securities Purchase Agreement with GS Capital, pursuant to which GS Capital purchased two 8% convertible redeemable junior subordinated promissory notes, each in the principal amount of $160,000. The first note (the “July 2017 GS Note”) was funded with cash and the second note (the “July 2017 GS Back-End Note”) was initially paid for by an offsetting promissory note issued by GS Capital to the Company (the “July 2017 GS Note Receivable”). The terms of the July 2017 GS Back-End Note required cash funding prior to any conversion thereunder. The July 2017 GS Note Receivable was due March 24, 2018, unless certain conditions were not met, in which case both the July 2017 GS Back-End Note and the July 2017 GS Note Receivable may both be cancelled. Both the July 2017 GS Note and the July 2017 GS Back-End Note matured on July 24, 2018 upon which any outstanding principal and interest is due and payable. The amounts cash funded plus accrued interest under both the July 2017 GS Note and the July 2017 GS Back-End Note are convertible into common stock of the Company at a conversion price equal to 62% of the lowest closing bid price of the common stock for the ten trading days prior to the conversion, subject to adjustment in certain events. On January 25, 2018, the Company received payment of the July 2017 GS Note Receivable in the amount of $160,000 that offset the July 2017 GS Back-End Note. Proceeds from the July 2017 GS Note Receivable of $8,000 were paid directly to legal fees resulting in net cash proceeds of $152,000 received by the Company. As a result, the July 2017 GS Back-End Note is now convertible. The July 2017 GS Note and the July 2017 GS Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company recorded a $98,065 put premium as each of the notes was funded.
As of June 30, 2018, the outstanding principal under the July 2017 GS Note and $8,169 of accrued interest was fully converted into shares of the Company’s common stock. As of June 30, 2018, $125,000 of principal under the July 2017 GS Back-End Note along with $3,420 in interest was converted. As of December 31, 2018, the remaining outstanding principal balance of $35,000 under the July 2017 GS Back-End Note along with $1,281 of accrued interest was fully converted (see Note 6 – Stockholders’ Deficit) resulting in full repayment of the note and a full reduction of the put premium.
September 21, 2017 Securities Purchase Agreement
On September 21, 2017, the Company entered into Securities Purchase Agreements, with GS Capital, dated as of September 12, 2017, pursuant to which GS Capital purchased two 8% convertible redeemable junior subordinated promissory notes, each in the principal amount of $160,000. The first note (the “September 2017 GS Note”) was funded with cash and the second note (the “September 2017 GS Back-End Note”) was initially paid for by an offsetting promissory note issued by GS Capital to the Company (the “September 2017 GS Note Receivable”). The terms of the September 2017 GS Back-End Note require cash funding prior to any conversion thereunder. The September 2017 GS Note Receivable was due March 24, 2018, unless certain conditions are not met, in which case both the September 2017 GS Back-End Note and the September 2017 GS Note Receivable may both be cancelled. Both the September 2017 GS Note and the September 2017 GS Back-End Note matured on September 12, 2018, upon which any outstanding principal and interest is due and payable. The amounts cash funded plus accrued interest under both the September 2017 GS Note and the September 2017 GS Back-End Note are convertible into common stock of the Company at a conversion price equal to 62% of the lowest closing bid price of the common stock for the ten trading days prior to the conversion, subject to adjustment in certain events. On February 27, 2018, the Company received payment of the September 2017 GS Note Receivable in the amount of $160,000 that offset the September 2017 GS Back-End Note. Proceeds from the September 2017 GS Note Receivable of $8,000 were paid directly to legal fees resulting in net cash proceeds of $152,000 received by the Company. As a result, the September 2017 GS Back-End Note is now convertible. The September 2017 GS Note and the September 2017 GS Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company recorded a $98,065 put premium as each of the notes was funded.
| F-19 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
As of June 30, 2018, $30,000 of principal under the September 2017 GS Note along with $1,289 in interest was converted. As of December 31, 2018, the remaining outstanding principal balance of $130,000 under the September 2017 GS Note along with $9,177 of accrued interest was fully converted (see Note 6 – Stockholders’ Deficit) resulting in full repayment of the note and a full reduction of the put premium. As of December 31, 2018, the outstanding principal balance of $160,000 under the September 2017 GS Back-End note along with $6,975 of accrued interest was fully converted (see Note 6 – Stockholders’ Deficit) resulting in full repayment of the note and a full reduction of the put premium.
March 23, 2018 Securities Purchase Agreement
On March 23, 2018, the Company entered into a securities purchase agreement with GS Capital, pursuant to which GS Capital purchased two 8% convertible redeemable junior subordinated promissory notes of the Company, each in the principal amount of $106,000. The first note (the “March 2018 GS Note”) was funded with cash and the second note (the “March 2018 GS Back-End Note”) was initially paid for by an offsetting promissory note issued by GS Capital to the Company (the “March 2018 GS Note Receivable”). The terms of the March 2018 GS Back-End Note require cash funding prior to any conversion thereunder. The March 2018 GS Note Receivable is due November 23, 2018, unless certain conditions are not met, in which case both the March 2018 GS Back-End Note and the March 2018 GS Note Receivable may both be cancelled. Both the March 2018 GS Note and the March 2018 GS Back-End Note mature on March 23, 2019, upon which any outstanding principal and interest is due and payable. The amounts cash funded plus accrued interest under both the March 2018 GS Note and the March 2018 GS Back-End Note are convertible into shares of common stock of the Company at a conversion price equal to 62% of the lowest closing bid price of the common stock for the ten trading days prior to the conversion, subject to adjustment in certain events. On May 31, 2018, the Company received payment of the March 2018 GS Note Receivable in the amount of $106,000 that offset the March 2018 GS Back-End Note. Proceeds from the March 2018 GS Note Receivable of $5,300 were paid directly to legal fees resulting in net cash proceeds of $100,700 received by the Company. As a result, the March 2018 GS Back-End Note is now convertible. The March 2018 GS Note and the March 2018 GS Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company recorded a $64,968 put premium as each of the notes was funded.
As of December 31, 2018, the outstanding principal balance of $106,000 under the March 2018 GS Note along with $2,765 of accrued interest was fully converted (see Note 6 – Stockholders’ Deficit) resulting in full repayment of the note and a full reduction of the put premium. As of December 31, 2018, the outstanding principal balance of $106,000 under the March 2018 GS Back-End note along with $4,740 of accrued interest was fully converted (see Note 6 – Stockholders’ Deficit) resulting in full repayment of the note and a full reduction of the put premium.
April 13, 2018 Securities Purchase Agreement
On April 13, 2018, the Company entered into a securities purchase agreement with GS Capital, pursuant to which GS Capital purchased two 8% unsecured convertible promissory notes (the “April 2018 GS Notes”) from the Company each in the principal amount of $150,000. The first note (the “April 2018 GS Note”) was funded with cash and the second note (the “April 2018 GS Back-End Note”) was initially paid for by an offsetting promissory note issued by GS Capital to the Company (the “April 2018 GS Note Receivable”). The terms of the April 2018 Back-End Note require cash funding prior to any conversion thereunder.
| F-20 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
Both the April 2018 GS Note and the April 2018 GS Back-End Note mature on April 13, 2019, upon which any outstanding principal and interest thereon is due and payable. The amounts cash funded plus accrued interest under both the April 2018 GS Note and the April 2018 GS Back-End Note are convertibles into shares of the Company’s common stock, at any time after October 13, 2018, at a conversion price for each share of common stock equal to 61% of the lowest closing bid price of the Company’s common stock for the ten prior trading days including the day upon which a notice of conversion is received by the Company from GS Capital, subject to adjustment in certain events. On September 12, 2018, the Company received payment of the April 2018 GS Note Receivable in the amount of $150,000 that offset the March 2018 GS Back-End Note. Proceeds from the March 2018 GS Note Receivable of $7,500 were paid directly to legal fees resulting in net cash proceeds of $142,500 received by the Company. Both the April 2018 GS Note and the April 2018 GS Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company recorded a $95,902 put premium as each of the notes were funded.
The Company has recorded $2,213 of accrued interest as of December 31, 2018 for the April 2018 GS Note. Total principal outstanding under the April 2018 GS Note as of December 31, 2018 was $85,000 as $65,000 was converted during the six months ended December 31, 2018 (see Note 6 – Stockholders’ Deficit).As of December 31, 2018, the outstanding principal balance of $150,000 under the April 2018 GS Back-End Note along with $1,606 of accrued interest was fully converted (see Note 6 – Stockholders’ Deficit) resulting in full repayment of the note and a full reduction of the put premium.
The April 2018 GS Note may be prepaid until 180 days from the issuance date with certain penalties.
The April 2018 GS Notes contain certain events of default, upon which principal and accrued interest will become immediately due and payable. In addition, upon an event of default, interest on the outstanding principal shall accrue at a default interest rate of 24% per annum, or if such rate is usurious or not permitted by current law, then at the highest rate of interest permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
October 2, 2018 Securities Purchase Agreement
Effective October 2, 2018, the Company entered into a securities purchase agreement with GS Capital, pursuant to which GS Capital purchased two 8% unsecured convertible redeemable notes (the “October 2018 GS Notes”) from the Company in the aggregate principal amount of $212,000, such principal and the interest thereon convertible into shares of the Company’s common stock. The purchase price of $106,000 of the first note (the “October 2018 GS Note”) was paid in cash by GS Capital on October 3, 2018. After payment of certain legal fees and expenses, net proceeds to the Company from the October 2018 GS Note totaled $100,700. The purchase price of $106,000 of the second note (the “October 2018 GS Back End Note”) was initially paid for by GS Capital issuing to the Company an offsetting $106,000 collateralized secured note (the “October 2018 GS Secured Note”). The terms of the October 2018 GS Back End Note require cash funding prior to any conversion thereunder, and such cash funding shall occur on or before June 2, 2019.
Both the October 2018 GS Note and the October 2018 GS Back End Note mature on October 2, 2019, upon which any outstanding principal and interest thereon is due and payable. The amounts cash funded plus accrued interest under both the October 2018 GS Note and the October 2018 GS Back End Note are convertibles into shares of the Company’s common stock, at any time after April 2, 2019, at a conversion price for each share of common stock equal to 61% of the lowest closing bid price of the Company’s common stock for the ten prior trading days including the day upon which a notice of conversion is received by the Company from GS Capital, subject to adjustment in certain events. GS Capital shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by GS Capital and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock. The October 2018 GS Note is treated as stock settled debt under ASC 480 and accordingly, the Company recorded a $67,771 put premium. The Company has recorded $4,182 of accrued interest and the total principal outstanding under the October 2018 GS Note was $106,000 as of December 31, 2018.
The October 2018 GS Note may be prepaid until 180 days from the issuance date with certain penalties. The October 2018 GS Back End Note may not be prepaid. However, in the event that the October 2018 GS Back End Note has not been cash paid and the October 2018 GS Note is redeemed within the first six months of issuance, the October 2018 GS Back-End Note will be deemed cancelled and of no further effect. The October 2018 GS Back End Note is not convertible until it is funded in cash on or before June 2, 2019, subject to certain restrictions. The Company has reserved 3,949,000 shares of its common stock for conversions under the October 2018 GS Note.
| F-21 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
The October 2018 GS Notes contain certain events of default, upon which principal and accrued interest will become immediately due and payable. In addition, upon an event of default, interest on the outstanding principal shall accrue at a default interest rate of 24% per annum, or if such rate is usurious or not permitted by current law, then at the highest rate of interest permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
The total principal amount outstanding under the above GS Capital financing agreements, specifically the April 13, 2018 and the October 2, 2018 agreements, was $191,000 as of December 31, 2018 and accrued interest thereunder totaled $6,395.
Consulting Agreement
On August 10, 2017, the Company entered into a consulting agreement, retroactive to May 16, 2017, with a certain consultant, pursuant to which the consultant agreed to provide certain consulting and business advisory services in exchange for a $310,000 junior subordinated convertible note. The note accrues interest at a rate of 10% per annum and is convertible into common stock at the lesser of $1.50 or 65% of the three lowest trades in the ten trading days prior to the conversion. The note was fully earned upon signing the agreement and matures on August 10, 2019. This note may not be prepaid without the written consent of the consultant. The Company accrued $155,000 related to this expense at June 30, 2017 and recorded the remaining $155,000 related to this expense in fiscal year 2018. Upon an event of default, principal and accrued interest will become immediately due and payable under the note. Additionally, upon an event of default the note would accrue interest at a default interest rate of 18% per annum or the highest rate of interest permitted by law. The consulting agreement had a three-month term and expired on August 16, 2017. An aggregate total of $578,212 of this note was bifurcated with the embedded conversion option recorded as a derivative liability at fair value. During the year ended June 30, 2018, the consultant converted $140,000 of principal and $10,764 of interest. During the six months ended December 31, 2018, the consultant converted an additional $161,000 of principal and $19,418 of interest (see Note 6 – Stockholders’ Deficit), such that the remaining principal outstanding and accrued interest under this note as of December 31, 2018 was $9,000 and $10,544, respectively.
Power Up Lending Group Financing Agreements
January 22, 2018 Securities Purchase Agreement
Effective January 22, 2018, the Company entered into a securities purchase agreement with Power Up Lending Group Ltd. (“Power Up”), pursuant to which Power Up purchased a convertible promissory note (the “January 2018 Power Up Note”) from the Company in the aggregate principal amount of $153,000, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Power Up. The transaction closed on January 25, 2018 and the Company received payment on January 29, 2018 in the amount of $153,000, of which $2,500 was paid directly toward legal fees and $500 to Power Up for due diligence fees resulting in net cash proceeds of $150,000.
The maturity date of the January 2018 Power Up Note is January 22, 2019. The January 2018 Power Up Note bears interest at a rate of 8% per annum, which interest may be paid by the Company to Power Up in shares of the Company’s common stock, but shall not be payable until the January 2018 Power Up Note becomes payable, whether at the maturity date or upon acceleration or by prepayment. An aggregate total of $180,251 of this note was bifurcated with the embedded conversion option recorded as a derivative liability at fair value.
Additionally, Power Up has the option to convert all or any amount of the principal face amount of the January 2018 Power Up Note, starting on July 21, 2018 and ending on the later of the maturity date and the date the Default Amount, which is an amount equal to 150% of an amount equal to the then outstanding principal amount of the January 2018 Power Up Note plus any interest accrued, is paid if an event of default occurs, for shares of the Company’s common stock at the then-applicable conversion price.
| F-22 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
The conversion price for the January 2018 Power Up Note shall be $0.065, subject to certain Market Price (as defined below) adjustment. If the Market Price is greater than or equal to $0.10, the conversion price shall be the greater of 65% of the Market Price (“Variable Conversion Price”) and $0.065. In the event Market Price is less than $0.10, the conversion price shall be the Variable Conversion Price. As defined in the January 2018 Power Up Note, the “Market Price” shall be the average of the lowest three closing bid prices during the ten day trading period prior to and including the day the Company receives a notice of conversion from Power Up on the electronic quotation system or applicable principal securities exchange or trading market or, if no closing bid price of such security is available in any of the foregoing manners, the average of the closing bid prices of any market makers for such security that are listed in the “pink sheets” during the ten prior trading days, including the day upon which the Company receives a notice of conversion from Power Up. Notwithstanding the foregoing, Power Up shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Power Up and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock. The January 2018 Power Up Note may be prepaid within 180 days of issuance with certain penalties.
During the six months ended December 31, 2018, the outstanding principal balance of $153,000 along with $6,120 of accrued interest was converted into shares of the Company’s common stock (See Note 6 – Stockholders’ Deficit) resulting in a full repayment of the note.
March 5, 2018 Securities Purchase Agreement
On March 5, 2018, the Company entered into a securities purchase agreement with Power Up, pursuant to which Power Up purchased a convertible promissory note (the “March 2018 Power Up Note”) from the Company in the aggregate principal amount of $53,000, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Power Up. The Company received payment on March 12, 2018 in the amount of $53,000, of which $2,500 was paid directly toward legal fees and $500 to Power Up for due diligence fees resulting in net cash proceeds of $50,000.
The maturity date of the March 2018 Power Up Note is March 5, 2019. The March 2018 Power Up Note shall bear interest at a rate of 8% per annum, which interest may be paid by the Company to Power Up in shares of the Company’s common stock, but shall not be payable until the March 2018 Power Up Note becomes payable, whether at the maturity date or upon acceleration or by prepayment. An aggregate total of $65,231 of this note was bifurcated with the embedded conversion option recorded as a derivative liability at fair value.
Additionally, Power Up has the option to convert all or any amount of the principal face amount of the March 2018 Power Up Note, starting on September 1, 2018 and ending on the later of the maturity date and the date the Default Amount, which is an amount equal to 150% of an amount equal to the then outstanding principal amount of the March 2018 Power Up Note plus any interest accrued, is paid if an event of default occurs, for shares of the Company’s common stock at the then-applicable conversion price.
The conversion price for the March 2018 Power Up Note shall be $0.065, subject to certain Market Price (as defined below) adjustment. If the Market Price is greater than or equal to $0.10, the conversion price shall be the greater of 65% of the Market Price (the “Variable Conversion Price”) and $0.065. In the event Market Price is less than $0.10, the conversion price shall be the Variable Conversion Price. As defined in the March 2018 Power Up Note, the “Market Price” shall be the average of the lowest three closing bid prices during the ten day trading period prior to and including the day the Company receives a notice of conversion from Power Up on the electronic quotation system or applicable principal securities exchange or trading market or, if no closing bid price of such security is available in any of the foregoing manners, the average of the closing bid prices of any market makers for such security that are listed in the “pink sheets” during the ten prior trading days, including the day upon which the Company receives a notice of conversion from Power Up. Notwithstanding the foregoing, Power Up shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Power Up and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock. The March 2018 Power Up Note may be prepaid within 180 days of issuance with certain penalties.
On August 28, 2018, the Company prepaid the outstanding principal balance of $53,000 and related accrued interest of $2,033 that was due under the March 5, 2018 Power Up Note and the note was deemed fully satisfied. The Company incurred a penalty in the amount of $20,362 as a result of the pre-payment.
| F-23 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
May 15, 2018 Securities Purchase Agreement
On May 15, 2018, the Company entered into a securities purchase agreement with Power Up, pursuant to which Power Up purchased a convertible promissory note (the “May 2018 Power Up Note”) from the Company in the aggregate principal amount of $53,000, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Power Up. The Company received payment on May 18, 2018 in the amount of $53,000, of which $2,500 was paid directly toward legal fees and $500 to Power Up for due diligence fees resulting in net cash proceeds of $50,000.
The maturity date of the May 2018 Power Up Note is May 5, 2019. The May 2018 Power Up Note shall bear interest at a rate of 8% per annum, which interest may be paid by the Company to Power Up in shares of common stock, but shall not be payable until the May 2018 Power Up Note becomes payable, whether at the maturity date or upon acceleration or by prepayment. An aggregate total of $33,744 of this note was bifurcated with the embedded conversion option recorded as a derivative liability at fair value.
Additionally, Power Up has the option to convert all or any amount of the principal face amount of the May 2018 Power Up Note, starting on November 11, 2018 and ending on the later of the maturity date and the date the Default Amount, which is an amount equal to 150% of an amount equal to the then outstanding principal amount of the May 2018 Power Up Note plus any interest accrued, is paid if an event of default occurs, for shares of the Company’s common stock at the then-applicable conversion price.
The conversion price for the May 2018 Power Up Note shall be $0.065, subject to certain Market Price (as defined below) adjustment. If the Market Price is greater than or equal to $0.10, the conversion price shall be the greater of 65% of the Market Price (“Variable Conversion Price”) and $0.065. In the event Market Price is less than $0.10, the conversion price shall be the Variable Conversion Price. As defined in the May 2018 Power Up Note, the “Market Price” shall be the average of the lowest three closing bid prices during the ten day trading period prior to and including the day the Company receives a notice of conversion from Power Up on the electronic quotation system or applicable principal securities exchange or trading market or, if no closing bid price of such security is available in any of the foregoing manners, the average of the closing bid prices of any market makers for such security that are listed in the “pink sheets” during the ten prior trading days, including the day upon which the Company receives a notice of conversion from Power Up. Notwithstanding the foregoing, Power Up shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Power Up and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock. The May 2018 Power Up Note may be prepaid by the Company within 180 days of issuance with certain penalties.
On November 7, 2018, the Company prepaid the outstanding principal balance of $53,000 and related accrued interest of $1,696 that was due under the May 2018 Power Up Note and the note was deemed fully satisfied. The Company incurred a penalty in the amount of $20,715 as a result of the pre-payment.
August 28, 2018 Securities Purchase Agreement
On August 28, 2018, the Company entered into a securities purchase agreement with Power Up, pursuant to which Power Up purchased a convertible promissory note (the “August 2018 Power Up Note”) from the Company in the aggregate principal amount of $53,000, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Power Up. The Company received payment on August 29, 2018 in the amount of $53,000, of which $2,500 was paid directly toward legal fees and $500 to Power Up for due diligence fees resulting in net cash proceeds of $50,000.
The maturity date of the August 2018 Power Up Note is August 28, 2019 (the “Maturity Date”). The August 2018 Power Up Note bears interest at a rate of 8% per annum, which interest may be paid by the Company to Power Up in shares of the Company’s common stock, but shall not be payable until the August 2018 Power Up Note becomes payable, whether at the Maturity Date or upon acceleration or by prepayment, as described below.
| F-24 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
Additionally, Power Up has the option to convert all or any amount of the principal face amount of the August 2018 Power Up Note, starting on February 24, 2019 at a conversion price of shall be $0.065, subject to certain Market Price (as defined below) adjustment. If the Market Price is greater than or equal to $0.10, the conversion price shall be the greater of 65% of the Market Price (“Variable Conversion Price”) and $0.065. In the event Market Price is less than $0.10, the conversion price shall be the Variable Conversion Price. As defined in the May 2018 Power Up Note, the “Market Price” shall be the average of the lowest three closing bid prices during the ten day trading period prior to and including the day the Company receives a notice of conversion from Power Up on the electronic quotation system or applicable principal securities exchange or trading market or, if no closing bid price of such security is available in any of the foregoing manners, the average of the closing bid prices of any market makers for such security that are listed in the “pink sheets” during the ten prior trading days, including the day upon which the Company receives a notice of conversion from Power Up. Notwithstanding the foregoing, Power Up shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Power Up and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock. An aggregate total of $396,380 of this note was bifurcated with the embedded conversion option recorded as a derivative liability at fair value (See Note 10 - Derivative Financial Instruments and Fair Value Measurements). The August 2018 Power Up Note may be prepaid by the Company until 180 days from the issuance date.
Upon an event of default, interest on the outstanding principal shall accrue at a default interest rate of 22% per annum. In the event that the Company fails to deliver to Power Up shares of common stock issuable upon conversion of principal or interest under the August 2018 Power Up Note within three business days of a notice of conversion by Power Up, the Company shall incur a penalty of $500, provided, however, that such fee shall not be due if the failure to deliver the shares is a result of a third party such as the transfer agent.
The Company has recorded $395 of accrued interest as of December 31, 2018 for the August 2018 Power Up Note. Total principal outstanding under the August 2018 Power Up Note as of December 31, 2018 was $53,000.
JSJ Investments, Inc. Financing Agreement
Effective June 26, 2018, the Company issued a convertible promissory note (the “June 2018 JSJ Note”) to JSJ Investments, Inc. (“JSJ”) in the aggregate principal amount of $113,000, with principal and the interest thereon convertible into shares of the Company’s common stock at the option of JSJ any time after 180 days of issuance. At the time of closing on June 27, 2018, JSJ deducted $3,000 from the principal payment due under the June 2018 JSJ Note to be applied to its legal expenses, such that the Company received aggregate net proceeds of $110,000 at closing.
The maturity date of the June 2018 JSJ Note is June 26, 2019, unless extended for up to one year at JSJ’s discretion (the “Maturity Date”). The June 2018 JSJ Note bears interest at a rate of 8% per annum, and after the maturity date shall compound quarterly.
Additionally, JSJ has the option to convert all or any amount of the principal face amount of the June 2018 JSJ Note, at any time beginning December 23, 2018, for shares of the Company’s common stock at a price equal to 65% of the lowest closing bid price (the “Closing Bid Price”) of the Company’s common stock as reported on the OTC Markets quotation system for the ten prior trading days, including the day upon which the Company receives a notice of conversion from JSJ (the “Conversion Price’). However, in the event that the Company’s common stock is restricted by the DTC for any reason, the Conversion Price shall be lowered to 50% of the lowest Closing Bid Price for the duration of such restriction. Notwithstanding the foregoing, JSJ shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by JSJ and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock. The June 2018 JSJ Note is treated as stock settled debt under ASC 480 and accordingly the Company recorded a $60,846 put premium.
The June 2018 JSJ Note may be prepaid until December 23, 2018. If the June 2018 JSJ Note is prepaid within 90 days of the issuance date, then the prepayment premium shall be 135% of the face amount plus any accrued interest; if the JSJ Note is prepaid after 90 days from the issuance date, but prior to 121 days from the issuance date, then the prepayment premium shall be 140% of the face amount plus any accrued interest; and if the June 2018 JSJ Note is prepaid after 120 days from the issuance date, but prior to 180 days from the issuance date, then the prepayment premium shall be 145% of the face amount plus any accrued interest.
| F-25 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
The Company shall at all times reserve a minimum of four times the number of shares required if all outstanding principal, and interest under the June 2018 JSJ Note would be fully converted, and JSJ may reasonably request increases from time to time to reserve share amounts.
The June 2018 JSJ Note contains certain events of default, including failure to timely issue shares upon receipt of a notice of conversion, failure to give at least 20 days notice of a reverse split, and failure to file all reports required to be filed by it with the SEC or the OTC Markets to remain a “Current Information” designated company, as well as certain customary events of default, including, among others, a breach of the covenants, insolvency, bankruptcy and failure by the Company to pay the principal and interest due under the June 2018 JSJ Note.
Upon an event of default, interest on the outstanding principal shall accrue at a default interest rate of 18% per annum or at the highest rate permitted by law. Amounts due under the June 2018 JSJ Note in the event of a default shall be based on the value of the underlying conversion shares and calculated off of the highest price of the Company’s common stock at any time between June 26, 2018 and the date of the event of default. In addition, for the first three occurrences of an event of default, the conversion discount shall be increased by 5% for each occurrence of a default.
On December 24, 2018, the Company prepaid the outstanding principal balance of $113,000 and related accrued interest of $4,508 that was due under the June 2018 JSJ Note and the note was deemed fully satisfied. The Company incurred a penalty in the amount of $51,380 as a result of the pre-payment.
Coventry Enterprises, LLC Financing Agreement
Effective June 29, 2018, the Company entered into a securities purchase agreement with Coventry Enterprises, LLC (“Coventry Enterprises”), pursuant to which Coventry Enterprises purchased two 8% unsecured convertible promissory notes from the Company in the aggregate principal amount of $200,000, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Coventry Enterprises.
The purchase price of $100,000 of the first note (the “July 2018 Coventry Note”) was paid in cash by Coventry Enterprises on July 2, 2018. After payment of certain legal fees and expenses, net proceeds to the Company from the First Note totaled $95,000. The purchase price of $100,000 of the second note (the “July 2018 Coventry Back-End Note”) was initially paid for by the issuance of an offsetting $100,000 collateralized secured note issued to Company by Coventry Enterprises (the “July 2018 Coventry Enterprises Note”). The terms of the July 2018 Coventry Back-End Note require cash funding prior to any conversion thereunder. The July 2018 Coventry Back-End Note is due February 29, 2019, unless certain conditions are not met, in which case both the July 2018 Coventry Back-End Note and the July 2018 Coventry Enterprise Note may both be cancelled. On September 6, 2018, the Company received payment of the July 2018 Coventry Enterprise Note in the amount of $100,000 that offset the July 2018 Coventry Back-End Note. Proceeds from the July 2018 Coventry Enterprise Note of $5,000 were paid directly to legal fees resulting in net cash proceeds of $95,000 received by the Company. As a result, the July 2018 Coventry Back-End Note is now convertible.
The maturity date of the July 2018 Coventry Note and the July 2018 Coventry Back-End Note is June 29, 2019. The outstanding principal amounts plus accrued interest under both the July 2018 Coventry Note and the July 2018 Coventry Back-End Note are convertible into shares of common stock of the Company at a conversion price equal to 61% of the lowest closing bid price of the Company’s common stock as reported on the exchange or quotation system on which the Company’s shares are then traded for the ten prior trading days including the day upon which a notice of conversion is received by the Company from Coventry Enterprises. Coventry Enterprises shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Coventry Enterprises and its affiliates, exceeds 9.9% of the outstanding shares of the Company’s common stock. Both the July 2018 Coventry Note and the July 2018 Coventry Back-End Notes are treated as stock settled debt under ASC 480 and accordingly the Company recorded a $63,934 put premium as each of the notes was funded. The Company has recorded $4,092 of accrued interest as of December 31, 2018 for the July 2018 Coventry Note and total principal outstanding as of December 31, 2018 was $71,250 as $28,750 was converted during the six months ended December 31, 2018 (see Note 6 – Stockholders’ Deficit). The Company has recorded $822 of accrued interest as of December 31, 2018 for the July 2018 Coventry Back-End Note. Total principal outstanding under the July 2018 Coventry Back-End Note as of December 31, 2018 was $24,700 as $75,300 was converted during the six months ended December 31, 2018 (see Note 6 – Stockholders’ Deficit). The July 2018 Coventry Note may be prepaid by the Company with certain penalties until 180 days from the issuance date. The July 2018 Coventry Back-End Note may not be prepaid by the Company.
| F-26 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
Upon an event of default, principal and accrued interest will become immediately due and payable under the notes. Additionally, upon an event of default, both notes will accrue interest at a default interest rate of 24% per annum or the highest rate of interest permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
The Company recorded $50,000 of debt discounts related to the above note issuances during the six months ended December 31, 2018. The debt discounts are being amortized over the term of the debt.
Amortization of all debt discounts for the six months ended December 31, 2018 and 2017 was $303,813 and $400,284, respectively.
See Note 11 – Subsequent Events for information about financing arrangements post December 31, 2018.
NOTE 6 – STOCKHOLDERS’ DEFICIT
On September 21, 2018, the Company filed a Certificate of Incorporation to increase the number of authorized shares of the Company’s common stock from 400,000,000 to 4,000,000,000, which was approved by the Company’s board of directors and holders of a majority of the Company’s voting stock on August 28, 2018.
Preferred Stock:
The total number of shares of preferred stock that the Company is authorized to issue is 1,500,005, $0.01 par value per share. These preferred shares have no rights to dividends, profit sharing or liquidation preferences.
Of the total preferred shares authorized, 500,000 have been designated as Series A Preferred Stock, $0.01 par value per share (“Series A Preferred Stock”), pursuant to the Certificate of Designation filed with the Secretary of State of the State of Delaware on December 9, 2014. James Nathanielsz, the Company’s Chief Executive Officer and Chief Financial Officer, beneficially owns all of the shares of Series A Preferred Stock via North Horizon Pty Ltd., which entitles him, as a holder of Series A Preferred Stock, to vote on all matters submitted or required to be submitted to a vote of the Company’s stockholders, except election and removal of directors, and each share of Series A Preferred Stock entitles him to two votes per share of Series A Preferred Stock. North Horizon Pty Ltd. is a Nathanielsz Family Trust. Mr. James Nathanielsz, the Chief Executive Officer, Chief Financial Officer and a director of our Company, has voting and investment power over these shares. 500,000 shares of Series A Preferred Stock are issued and outstanding as of December 31, 2018.
Of the total preferred shares authorized, pursuant to the Certificate of Designation filed with the Secretary of State of the State of Delaware on June 16, 2015, up to five shares have been designated as Series B Preferred Stock, $0.01 par value per share (“Series B Preferred Stock”). Each holder of outstanding shares of Series B Preferred Stock is entitled to voting power equivalent to the number of votes equal to the total number of shares of common stock outstanding as of the record date for the determination of stockholders entitled to vote at each meeting of stockholders of the Company and entitled to vote on all matters submitted or required to be submitted to a vote of the stockholders of the Company. One share of Series B Preferred Stock is issued and outstanding as of December 31, 2018. Mr. Nathanielsz directly beneficially owns such one share of Series B Preferred Stock.
Common Stock:
Shares issued for conversion of convertible debt
During the three months ended September 30, 2018, the Company issued 129,142,548 shares of its common stock at an average conversion price of $0.01, ranging from $0.002 to $0.04, as a result of the conversion of principal and interest in the aggregate amount of $1,413,317 underlying certain outstanding convertible notes converted during such period.
| F-27 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
During the three months ended December 31, 2018, the Company issued 63,842,216 shares of its common stock at an average conversion price of $0.02, ranging from $0.008 to $0.04, as a result of the conversion of principal and interest in the aggregate amount of $1,095,100 underlying certain outstanding convertible notes converted during such period.
The Company has 806,210,142 shares of its common stock reserved for future issuances based on lender reserve requirements pursuant to underlying financing documents at December 31, 2018.
Shares issued for services
On December 6, 2018, the Company entered into an agreement with a certain consultant to provide services over a six-month period beginning November 1, 2018 and ending May 1, 2019 in exchange for 2,000,000 shares of the Company’s common stock. On December 27, 2018, the Company issued the 2,000,000 shares of the Company’s common stock valued at $0.02 per share to the consultant, or $39,000, which will be amortized over the term of the agreement. The Company recorded $13,144 of consulting expense with respect to such shares of its common stock during the six months ended December 31, 2018.
On November 20, 2018, the Board of Directors authorized the issue of 1,000,000 shares of the Company’s common stock in connection with certain legal services provided to the Company. On November 28, 2018, the Company issued the 1,000,000 shares of its common stock valued at $0.03 or $30,000.
October 5, 2018 Equity Purchase Agreement
On October 5, 2018 (the “Closing Date”), the Company entered into an Equity Purchase Agreement (the “Purchase Agreement”) with an institutional accredited investor (the “Investor”) pursuant to which the Investor committed to purchase up to $10,000,000 (the “Maximum Amount”) of the Company’s common stock (the “Financing”). On the Closing Date, the Company issued 3,850,597 shares of the Company’s common stock to the Investor as a commitment fee (the “Commitment Shares”), at a fair market value of $0.08 or $318,059, which was recorded as deferred offing costs and will be amortized as a percentage of the Maximum Amount on a pro-rata conversion amount. Additionally, the proceeds received from the first put notice were net of $15,000 in legal fees and were recorded as deferred offering costs. Total amortization expense for the six months ended was $19,136. The Commitment Shares are subject to a lock-up/leak-out limitation as described below. In connection with the Financing, on the Closing Date, the Company and the Investor also entered into a Registration Rights Agreement (the “Registration Rights Agreement”, and together with the Purchase Agreement, the “Transaction Documents”). The Company will receive net proceeds from the sale of the Put Shares directly to the Investor pursuant to the Purchase Agreement, however, the Company will not receive any proceeds from the resale of the Put Shares by the Investor thereafter.
Upon filing and effectiveness of the Company’s Registration Statement on Form S-1, which was declared effective by the SEC on October 30, 2018, and provided other closing conditions are met, from time to time over the term of the Purchase Agreement, the Company shall have the right, but not the obligation, to direct the Investor to purchase shares of the Company’s common stock (the “Put Shares”) in a maximum amount of $1,000,000, provided that the number of Put Shares shall not exceed 250% of the Average Daily Trading Volume (as defined in the Purchase Agreement). At any time and from time to time during the 3-year term of the Purchase Agreement (the “Commitment Period”), the Company may deliver a notice to Investor (the “Put Notice”) and shall deliver the Put Shares to Investor via DWAC (as defined in the Purchase Agreement) within two trading days. The purchase price (the “Purchase Price”) for the Put Shares shall equal 87.5% of the one lowest daily volume weighted average price on the Principal Market (as defined in the Purchase Agreement) (as reported by Bloomberg Finance L.P.) during the five trading days immediately following the date the Investor receives the Put Shares via DWAC associated with the applicable Put Notice (the “Valuation Period”). The closing of a Put Notice shall occur within one trading day following the end of the respective Valuation Period, whereby (i) the Investor Shall deliver the Investment Amount (as defined below) to the Company by wire transfer of immediately available funds and (ii) Investor shall return surplus Put Shares if the value of the Put Shares delivered to the Investor causes the Company to exceed the maximum commitment amount. The Company shall not deliver another Put Notice to Investor within ten trading days of a prior Put Notice. The “Investment Amount” means the aggregate Purchase Price for the Put Shares purchased by the Investor, minus clearing costs payable to the Investor’s broker or to the Company’s transfer agent for the issuance of the Put Shares (the “Clearing Costs”).
| F-28 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
The right of the Company to issue and sell the Put Shares to the Investor is subject to the satisfaction of certain closing conditions, including, but not limited to, (i) the Company’s Registration Statement on Form S-1 registering for resale by the Investor of the Put Shares and Commitment Shares continuing to be effective as was declared by the U.S. Securities and Exchange Commission (the “SEC”) on October 30, 2018, (ii) accuracy of the Company’s representations and warranties, (iii) the Company’s performance under the Purchase Agreement in all material respects, (iv) no suspension of trading or delisting of the Company’s common stock, (v) limitation of the Investor’s beneficial ownership to no more than 9.99%, (vi) the Company maintaining its DWAC-eligible status, (vii) the Company maintaining a sufficient share reserve, and (viii) the minimum pricing for the Put Shares must exceed $0.0001.
Pursuant to the terms of the Registration Rights Agreement, the Company filed the Registration Statement on October 17, 2018 and the Registration Statement was declared effective by the SEC on October 30, 2018.
The Investor agreed, for a period of 180 days from the Closing Date, not to sell, on any given day, a number of Commitment Shares that exceeds the greater of (i) 5% of the average daily trading volume of the Company’s shares of common stock for the period ended one trading day prior to the date of such sale, as reported on the Principal Market; and (ii) such number of Commitment Shares that equals (x) $5,000, divided by, (y) the closing price of the Company’s shares of common stock one trading day prior to the date of such sale, as reported on the Principal Market.
Effective as of the Closing Date, the Company reserved 462,071,621 shares of its common stock from its authorized and unissued shares of common stock to provide for all issuances of shares of common stock under the Transaction Documents (in the event that the Company issues and sells the Put Shares up to the Maximum Amount) and is required to reserve and keep available out of its authorized and unissued shares of common stock a number of shares of common stock at least three times the number of shares of common stock obtained by dividing the remaining balance on the maximum commitment amount by the Purchase Price.
While the Company has the obligation to maintain such reserve while the Purchase Agreement is effective, the Company does not have the obligation to sell any Put Shares to the Investor. Neither the Investor, nor any affiliate of the Investor acting on its behalf or pursuant to any understanding with it, will execute any short sales during the period from the date hereof to the end of the Commitment Period.
During the six months ended December 31, 2018, the Company issued 27,000,000 shares of its common stock at an average price per share of $0.02, ranging from $0.01 to $0.04 as a result of executing nine Put Notices. As of December 31, 2018, the Company had received gross proceeds of $405,773 and recorded a balance of $168,787 in other current assets for proceeds owed related to the execution of these Put Notices.
Warrants:
On August 29, 2018, the Company received payment of $39 AUD for the exercise of a warrant for 12,000 shares of the Company’s common stock and issued such shares as a result of the exercise.
On December 2, 2018, a total of 104,000 warrants expired.
No warrants were issued during the six months ended December 31, 2018.
As of December 31, 2018, there were 29,517 warrants outstanding and exercisable with expiration dates commencing May 2010 and continuing through November 2020, with a weighted average exercise price per share of $9.53.
Options:
As of December 31, 2018, the Company had entered into agreements to grant options to purchase 572,000 shares of its common stock, with a weighted average exercise price per share of $7.50.
No stock options were issued during the six months ended December 31, 2018.
| F-29 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
NOTE 7 – COMMITMENTS AND CONTINGENCIES
Legal Matters
From time to time, the Company may be involved in litigation relating to claims arising out of the Company’s operations in the normal course of business. As of December 31, 2018, there were no pending or threatened lawsuits that could reasonably be expected to have a material effect on the results of the Company’s operations or business.
IRS Liability
As part of its requirement for having a foreign operating subsidiary, the Company is required to file an informational Form 5471 to the Internal Revenue Service (the “IRS”), which is a form that explains the nature of the relationship between the foreign subsidiary and the parent company. From 2012 through the 2014, the Company did not file this form in a timely manner. As a result of the non-timely filings, the Company incurred a penalty from the IRS in the amount of $10,000 per year, or $30,000 in total, plus accrued interest. The Company recorded the penalties for all three years during the year ended June 30, 2018 and is negotiating a payment plan. The Company is current on all subsequent filings.
Operating Agreements
In November 2009, the Company entered into a commercialization agreement with the University of Bath (UK) (the “University”) whereby the Company and the University co-owned the intellectual property relating to the Company’s pro-enzyme formulations. In June 2012, the Company and the University entered into an assignment and amendment whereby the Company assumed full ownership of the intellectual property while agreeing to pay royalties of 2% of net revenues to the University. Additionally, the Company agreed to pay 5% of each and every license agreement subscribed for. The contract is cancellable at any time by either party. To date, no amounts are owed under the agreement.
Operating Leases
On May 4, 2016, the Company entered into a new five-year operating lease agreement with a Horizon Pty Ltd., a related party, of which Mr. Nathanielsz, our CEO, CFO and a director, and his wife are owners and directors, with monthly rent of $3,300 AUD or $2,325 USD, inclusive of GST (See Note 8 – Related Party Transactions).
Future minimum operating lease commitments consisted of the following at December 31, 2018:
| Fiscal Year Ended June 30, | Amount (USD) | |||
| Remainder 2019 | $ | 13,951 | ||
| 2020 | $ | 27,900 | ||
| 2021 | $ | 27,900 | ||
Rent expense for the six months ended December 31, 2018 and 2017 was $14,527 and $15,729, respectively.
| F-30 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
Amatsigroup Agreement
The Company entered into a Manufacturing Services Agreement (the “MSA”) and Quality Assurance Agreement (the “QAA”), each with an effective date of August 12, 2016, with Amatsigroup NV (“Amatsigroup”), formerly known as Q-Biologicals, NV, a contract manufacturing organization located in Belgium. Pursuant to the MSA, Amatsigroup produces certain drug substances and products containing certain enzymes for the Company at its facility in Belgium. The Company uses these substances and products for development purposes, including but not limited to future clinical trials. The MSA contemplates payment to Amatsigroup pursuant to a pre-determined fee schedule based on the completion of certain milestones that depend on our manufacturing requirements and final batch yield. The Company anticipates that its payments to Amatsigroup under the MSA will range between $2.5 million and $5.0 million over three years, when the finished drug product is manufactured and released for clinical trials. The Company has spent a total of $1,689,146 of costs to date under this contract of which $1,639,192 was expensed in prior years and $49,854 was expensed in the six months ended December 31, 2018. The MSA shall continue for a term of three years unless extended by mutual agreement in writing. The Company can terminate the MSA early for any reason upon the required notice period, however, in such event, the pre-payment paid upon signing the MSA is considered non-refundable. Each party to the MSA shall have the right to terminate the MSA by written notice to the other party if the other party commits a material breach of the MSA (subject to a 30-day cure period). The QAA sets forth the parties respective obligations and responsibilities relating to the manufacturing and testing of the products under the MSA. The agreements with Amatsigroup contain certain customary representations, warranties and limitations of liabilities, and confidentiality and indemnity obligations.
Investment Banking Agreement
On February 23, 2018, the Company entered into an agreement with an effective date of February 14, 2018 with a certain investment bank, pursuant to which the Company retained the investment bank as its placement agent. As of December 31, 2018, no funds have been raised pursuant to this agreement and the agreement expired on its terms.
Collaboration Agreement
On September 13, 2018, the Company entered into a two-year collaboration agreement with the University of Jaen (the “University”) to provide certain research services to the Company. In consideration of such services, the Company agreed to pay the University approximately 52,000 Euros ($59,508 USD) in year one and a maximum of 40,000 Euros ($45,775 USD) in year two. Additionally, in exchange for full ownership of the intellectual property the Company agreed to pay royalties of 2% of net revenues to the University.
NOTE 8 – RELATED PARTY TRANSACTIONS
Since its inception, the Company has conducted transactions with its directors and entities related to such directors. These transactions have included the following:
As of December 31, 2018 and June 30, 2018, the Company owed a current and a former director a total of $52,140 and $54,753, respectively, for money loaned to the Company throughout the years. The total loans balance owed at December 31, 2018 and June 30, 2018 is not interest bearing (See Note 4 – Loans and Note Payable).
As of December 31, 2018 and June 30, 2018, the Company owed its former director a total of $31,329 and $32,898, respectively, related to expenses paid on behalf of the Company related to corporate startup costs and intellectual property (See Note 3 – Due to Former Director – Related Parties).
Effective May 5, 2016, the Company entered into an agreement for the lease of its principal executive offices with North Horizon Pty Ltd., a related party, of which Mr. Nathanielsz, our CEO, CFO and a director, and his wife are owners and directors. The lease has a five-year term and provides for annual rental payments of $39,600 AUD or $27,902 USD, which includes $3,600 AUD or $2,537 USD of goods and service tax for total payments of $198,000 AUD or $139,511 USD during the term of the lease. As of December 31, 2018, total payments of $99,000 AUD or $69,755 USD remain on the lease.
The Company and Mr. Nathanielsz entered into an employment agreement as of February 25, 2015 (the “Nathanielsz Employment Agreement”) setting forth the terms and conditions of Mr. Nathanielsz employment as the Company’s President and Chief Executive Officer. The Nathanielsz Employment Agreement expired on February 25, 2018; however, the term of the Nathanielsz Employment Agreement automatically renews for successive one-year periods unless either party provides 30 days’ prior written notice of its intent not to renew. The Nathanielsz Employment Agreement continues in effect as of December 31, 2018. The Nathanielsz Employment Agreement provides Mr. Nathanielsz with a base salary of $25,000 AUD per month ($300,000 AUD annually or $211,380 USD) and a monthly contribution to Mr. Nathanielsz’s pension equal to 9.5% of his monthly salary. Mr. Nathanielsz has the ability to convert any accrued but unpaid salary into common stock at the end of each fiscal year at a conversion price to be determined by Mr. Nathanielsz and the Company, which will in no event be lower than par value or higher than the closing bid price on the date of conversion .The Company also agreed to pay Mr. Nathanielsz an annual discretionary bonus in an amount up to 200% of his annual base salary, which bonus shall be determined by the board of directors and based upon the performance of the Company. On March 16, 2018, the Company’s board of directors approved an increase of $100,000 AUD ($70,460 USD) in Mr. Nathanielsz’ annual base salary, from $300,000 AUD ($211,380 USD) to $400,000 AUD ($281,840 USD), effective immediately.
| F-31 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
Mr. Nathanielsz’s wife, Sylvia Nathanielsz, is and has been a non-executive part-time employee of our Company since October 2015. Effective February 1, 2018. Mrs. Nathanielsz receives an annual salary of $84,552 and is entitled to customary benefits.
Pursuant to a February 25, 2016 board resolution, James Nathanielsz shall be paid $4,481 AUD ($3,157 USD), on a monthly basis for the purpose of acquiring and maintaining an automobile. For the six months ended December 31, 2018, a total of $18,944 in payments have been made with regards to the board resolution.
Pursuant to the approval of the board of directors, on March 16, 2018, Mr. Nathanielsz was granted a $300,000 AUD bonus for accomplishments achieved while serving as the Company’s chief executive officer during the fiscal years ended June 30, 2018. A total of $80,046 AUD in payments were made in the year ended June 30, 2018. During the six months ended December 31, 2018, an additional $130,000 AUD ($91,588 USD) was paid. The balance of the accrued bonus as of December 31, 2018 was $89,954 AUD ($63,382 USD).
NOTE 9 – CONCENTRATIONS AND RISKS
Concentration of Credit Risk
The Company maintains its cash in banks and financial institutions in Australia. Bank deposits in Australian banks are uninsured. The Company has not experienced any losses in such accounts through December 31, 2018.
Receivable Concentration
As of December 31, 2018 and June 30, 2018, the Company’s receivables were 100% related to reimbursements on GST taxes paid.
Patent and Patent Concentration
The Company has filed multiple patent applications relating to its lead product, PRP. The Company’s lead patent application has been granted and remains in force in the United States, Belgium, Czech Republic, Denmark, France, Germany, Ireland, Italy, Netherlands, Portugal, Spain, Sweden, Switzerland, Liechtenstein, Turkey, United Kingdom, Australia, China, Japan, Indonesia, Israel, New Zealand, Singapore, Malaysia, South Africa, Mexico, Republic of Korea and India. In Brazil and Canada, the patent application remains under examination.
In 2016 and early 2017, we filed other patent applications. Three applications were filed under the Patent Cooperation Treaty (the “PCT”). The PCT assists applicants in seeking patent protection by filing one international patent application under the PCT, applicants can simultaneously seek protection for an invention in over 150 countries. Once filed, the application is placed under the control of the national or regional patent offices, as applicable, in what is called the national phase. One of the PCT applications filed in November 2016, entered national phase in July 2018 and another PCT application is currently entering national phase in August 2018. A third PCT application entered the national phase in October 2018.
Further patent applications are expected to be filed to capture and protect additional patentable subject matter based on the Company’s field of technology relating to pharmaceutical compositions of proenzymes for treating cancer.
Foreign Operations
As of December 31, 2018 and June 30, 2018, the Company’s operations are based in Camberwell, Australia, however the majority of research and development is being conducted in the European Union.
| F-32 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
On July 22, 2016, the Company formed a wholly owned subsidiary, Propanc (UK) Limited under the laws of England and Wales for the purpose of submitting an orphan drug application with the European Medicines Agency as a small and medium-sized enterprise. As of December 31, 2018, there has been no activity within this entity.
NOTE 10 - DERIVATIVE FINANCIAL INSTRUMENTS AND FAIR VALUE MEASUREMENTS
Derivative Financial Instruments:
The Company applies the provisions of ASC 815-40, Contracts in Entity’s Own Equity, under which convertible instruments and warrants, which contain terms that protect holders from declines in the stock price (reset provisions), may not be exempt from derivative accounting treatment. As a result, warrants and embedded conversion options in convertible debt are recorded as a liability and are revalued at fair value at each reporting date. If the fair value of the warrants exceeds the face value of the related debt, the excess is recorded as change in fair value in operations on the issuance date. The Company had $62,000 of convertible debt, which is treated as derivative instruments outstanding at December 31, 2018.
The Company calculates the estimated fair values of the liabilities for derivative instruments using the Binomial Trees Method. The closing price of the Company’s common stock at December 31, 2018, the last trading day of the quarter, was $0.0193. Volatility, expected remaining term and risk free interest rates used to estimate the fair value of derivative liabilities at December 31, 2018 are indicated in the table that follows. The expected term is equal to the remaining term of the warrants or convertible instruments and the risk free rate is based upon rates for treasury securities with the same term.
Convertible Debt
Initial Valuations (on
new derivative December 31, 2018) | December 31, 2018 | |||||||
| Volatility | 294.92 | % | 393.04% – 409.24 | % | ||||
| Expected Remaining Term (in years) | 1 | .61 - .66 | ||||||
| Risk Free Interest Rate | 2.47 | % | 2.56 | % | ||||
| Expected dividend yield | None | None | ||||||
Fair Value Measurements:
The Company measures and reports at fair value the liability for derivative instruments. The fair value liabilities for price adjustable warrants and embedded conversion options have been recorded as determined utilizing the Binomial Trees model. The following tables summarize the Company’s financial assets and liabilities measured at fair value on a recurring basis as of December 31, 2018:
| Balance
at December 31, 2018 | Quoted Prices in Active Markets for Identical Assets | Significant Other Observable Inputs | Significant Unobservable Inputs | |||||||||||||
| (Level 1) | (Level 2) | (Level 3) | ||||||||||||||
| Embedded conversion option liabilities | $ | 66,490 | $ | — | $ | — | $ | 66,490 | ||||||||
| Total | $ | 66,490 | $ | — | $ | — | $ | 66,490 | ||||||||
| F-33 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three and Six Months Ended December 31, 2018 and 2017
(unaudited)
The following is a roll forward for the six months ended December 31, 2018 of the fair value liability of price adjustable derivative instruments:
| Fair Value of | ||||
| Liability for | ||||
| Derivative | ||||
| Instruments | ||||
| Balance at June 30, 2018 | $ | 371,532 | ||
| Reductions due to conversions | (1,388,764 | ) | ||
| Reductions due to repayment of debt | (897,924 | ) | ||
| Initial fair value of embedded conversion option derivative liability recorded as debt discount | 50,000 | |||
| Initial fair value of embedded conversion option derivative liability recorded as change in fair value of embedded conversion option | 346,380 | |||
| Change in fair value included in statements of operations | 1,585,266 | |||
| Balance at December 31, 2018 | $ | 66,490 | ||
NOTE 11 – SUBSEQUENT EVENTS
Note Conversions
From January 1, 2019 through the date of this Quarterly Report, the Company issued 31,044,271 shares of its common stock at an average conversion price of $0.01, ranging from $0.009 to $0.01, as a result of the conversion of principal and interest in the aggregate amount of $279,708 underlying certain outstanding convertible notes converted during such period.
Put Notices
From January 1, 2019 through the date of this Quarterly Report, the Company issued 29,600,000 shares of its common stock at an average price per share of $0.01 and has received gross proceeds of $277,401 and anticipates the receipt of approximately $120,000 in gross proceeds as a result of executing the last submitted put notice.
Investment Banking Agreement
On February 4, 2019, the Company entered into a letter agreement with a certain investment bank (the “Investment Bank”), pursuant to which the Company retained the Investment Bank as its exclusive placement agent through May 31, 2019. In the event of the closing of an offering during such period (or the tail period after, if applicable) the Investment Bank would receive a percentage of the proceeds in cash and a percentage of the shares of common stock issued by the Company in the offering as warrants.
| F-34 |

Report of Independent Registered Public Accounting Firm
To the Stockholders’ and the Board of Directors of:
Propanc Biopharma, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Propanc Biopharma, Inc. and Subsidiaries (the “Company”) as of June 30, 2018 and 2017, the related consolidated statements of operations and comprehensive income (loss), changes in stockholders’ deficit, and cash flows, for each of the two years in the period ended June 30, 2018, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the consolidated financial position of the Company as of June 30, 2018 and 2017, and the consolidated results of its operations and its cash flows for each of the two years in the period ended June 30, 2018, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company has a net loss and cash used in operations of $7,039,155 and $2,177,645, respectively, in 2018 and has a working capital deficit, stockholders’ deficit and accumulated deficit of $6,762,417, $6,751,920 and $45,282,678, respectively, at June 30, 2018. These matters raise substantial doubt about the Company’s ability to continue as a going concern. Management’s Plan in regards to these matters is also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
| /s/ SALBERG & COMPANY, P.A. | |
| SALBERG & COMPANY, P.A. | |
| We have served as the Company’s auditor since 2011. | |
| Boca Raton, Florida | |
| September 14, 2018 |
2295 NW Corporate Blvd., Suite 240 • Boca Raton, FL 33431
Phone: (561) 995-8270 • Toll Free: (866) CPA-8500 • Fax: (561) 995-1920
www.salbergco.com • info@salbergco.com
Member National Association of Certified Valuation Analysts • Registered with the PCAOB
Member CPA Connect with Affiliated Offices Worldwide • Member Center for Public Company Audit Firms
| F-35 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
CONSOLIDATED BALANCE SHEETS
| June 30, 2018 | June 30, 2017 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | $ | 19,921 | $ | 69,043 | ||||
| GST tax receivable | 6,257 | 8,111 | ||||||
| Prepaid expenses and other current assets | 34,712 | 4,822 | ||||||
| TOTAL CURRENT ASSETS | 60,890 | 81,976 | ||||||
| Security deposit - related party | 2,220 | 2,303 | ||||||
| Property and equipment, net | 8,277 | 10,790 | ||||||
| TOTAL ASSETS | $ | 71,387 | $ | 95,069 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable | $ | 1,157,369 | $ | 483,513 | ||||
| Accrued expenses and other payables | 364,404 | 477,347 | ||||||
| Convertible notes and related accrued interest, net of discounts and premiums | 4,699,299 | 3,479,845 | ||||||
| Loans payable | - | 2,303 | ||||||
| Embedded conversion option liabilities | 371,532 | 877,403 | ||||||
| Warrant derivative liability | - | 3,769 | ||||||
| Due to directors - related parties | 32,898 | 35,204 | ||||||
| Loans from directors and officer - related parties | 54,753 | 56,802 | ||||||
| Employee benefit liability | 143,052 | 120,634 | ||||||
| TOTAL CURRENT LIABILITIES | 6,823,307 | 5,536,820 | ||||||
| Commitments and Contingencies (See Note 9) | ||||||||
| STOCKHOLDERS’ DEFICIT: | ||||||||
| Preferred stock, 1,500,005 shares authorized, $0.01 par value: | ||||||||
| Series A preferred stock, $0.01 par value; 500,000 shares authorized; 500,000 and 500,000 shares issued and outstanding as of June 30, 2018 and June 30, 2017, respectively | 5,000 | 5,000 | ||||||
| Series B preferred stock, $0.01 par value; 5 shares authorized; 1 and 1 share issued and outstanding as of June 30, 2018 and June 30, 2017, respectively | - | - | ||||||
| Common stock, $0.001 par value; 400,000,000 shares authorized; 46,429,423 and 4,578,284 shares issued; 46,404,945 and 4,553,806 outstanding as of June 30, 2018 and June 30, 2017, respectively | 46,429 | 4,578 | ||||||
| Additional paid-in capital | 38,167,877 | 32,980,420 | ||||||
| Accumulated other comprehensive income (loss) | 357,929 | (141,749 | ) | |||||
| Accumulated deficit | (45,282,678 | ) | (38,243,523 | ) | ||||
| Treasury stock | (46,477 | ) | (46,477 | ) | ||||
| TOTAL STOCKHOLDERS’ DEFICIT | (6,751,920 | ) | (5,441,751 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | $ | 71,387 | $ | 95,069 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-36 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME (LOSS)
| Years Ended June 30, | ||||||||
| 2018 | 2017 | |||||||
| REVENUE | ||||||||
| Revenue | $ | - | $ | - | ||||
| OPERATING EXPENSES | ||||||||
| Administration expenses | 2,103,684 | 4,739,431 | ||||||
| Occupancy expenses | 30,521 | 28,992 | ||||||
| Research and development | 1,825,728 | 971,769 | ||||||
| TOTAL OPERATING EXPENSES | 3,959,933 | 5,740,192 | ||||||
| LOSS FROM OPERATIONS | (3,959,933 | ) | (5,740,192 | ) | ||||
| OTHER INCOME (EXPENSE) | ||||||||
| Interest expense | (2,789,196 | ) | (3,202,774 | ) | ||||
| Interest income | 87 | 685 | ||||||
| Change in fair value of derivative liabilities | (7,612 | ) | 820,153 | |||||
| Loss on debt settlements, net | (18,585 | ) | (195,650 | ) | ||||
| Gain on extinguishment of debt, net | 251,392 | - | ||||||
| Foreign currency transaction gain (loss) | (694,614 | ) | 144,605 | |||||
| TOTAL OTHER INCOME (EXPENSE) | (3,258,528 | ) | (2,432,981 | ) | ||||
| LOSS BEFORE TAXES | (7,218,461 | ) | (8,173,173 | ) | ||||
| TAX BENEFIT | 179,306 | 305,673 | ||||||
| NET LOSS | $ | (7,039,155 | ) | $ | (7,867,500 | ) | ||
| BASIC AND DILUTED NET LOSS PER SHARE | $ | (0.36 | ) | $ | (2.24 | ) | ||
| BASIC AND DILUTED WEIGHTED AVERAGE SHARES OUTSTANDING | 19,690,643 | 3,508,532 | ||||||
| NET LOSS | $ | (7,039,155 | ) | $ | (7,867,500 | ) | ||
| OTHER COMPREHENSIVE INCOME (LOSS) | ||||||||
| Unrealized foreign currency translation gain (loss) | 499,678 | (273,013 | ) | |||||
| TOTAL OTHER COMPREHENSIVE INCOME (LOSS) | 499,678 | (273,013 | ) | |||||
| TOTAL COMPREHENSIVE INCOME (LOSS) | $ | (6,539,477 | ) | $ | (8,140,513 | ) | ||
The accompanying notes are an integral part of these consolidated financial statements.
| F-37 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT
FOR THE YEARS ENDED JUNE 30, 2018 AND 2017
| Preferred Stock | Accumulated | |||||||||||||||||||||||||||||||||||||||||||
| Series A | Series B | Common Stock | Additional | Other | Total | |||||||||||||||||||||||||||||||||||||||
| No. of Shares | Value | No. of Shares | Value | No. of Shares | Value | Paid-in Capital | Accumulated Deficit | Treasury Stock | Comprehensive Income (Loss) | Stockholders' Deficit | ||||||||||||||||||||||||||||||||||
| Balance at June 30, 2016 | 500,000 | $ | 5,000 | 1 | $ | - | 2,914,465 | $ | 2,914 | $ | 27,671,552 | $ | (30,376,023 | ) | $ | - | $ | 131,264 | $ | (2,565,293 | ) | |||||||||||||||||||||||
| Issuance of common stock for conversion of convertible debt and accrued interest | - | - | - | - | 1,234,910 | 1,235 | 1,405,501 | - | - | - | 1,406,736 | |||||||||||||||||||||||||||||||||
| Reclassification of premium upon debt conversion | - | - | - | - | - | - | 266,287 | - | - | - | 266,287 | |||||||||||||||||||||||||||||||||
| Settlement of accounts payable for shares of common stock | - | - | - | - | 16,667 | 17 | 49,983 | - | - | - | 50,000 | |||||||||||||||||||||||||||||||||
| Loss on settlement of debt | - | - | - | - | - | - | 158,150 | - | - | - | 158,150 | |||||||||||||||||||||||||||||||||
| Issuance of stock for services | - | - | - | - | 307,480 | 307 | 459,637 | - | - | - | 459,944 | |||||||||||||||||||||||||||||||||
| Stock option expense | - | - | - | - | - | - | 1,686,444 | - | - | - | 1,686,444 | |||||||||||||||||||||||||||||||||
| Cancellation of shares for convertible notes payable | - | - | - | - | (50,000 | ) | (50 | ) | (112,450 | ) | - | - | - | (112,500 | ) | |||||||||||||||||||||||||||||
| Warrant modification expense | - | - | - | - | - | - | 21,007 | - | - | - | 21,007 | |||||||||||||||||||||||||||||||||
| Relative fair value of warrants issued with convertible debt | - | - | - | - | - | - | 910,178 | - | - | - | 910,178 | |||||||||||||||||||||||||||||||||
| Exercise of warrants | - | - | - | - | 154,762 | 155 | 464,131 | - | - | - | 464,286 | |||||||||||||||||||||||||||||||||
| Purchase of treasury stock | - | - | - | - | - | - | - | - | (46,477 | ) | - | (46,477 | ) | |||||||||||||||||||||||||||||||
| Foreign currency translation loss | - | - | - | - | - | - | - | - | - | (273,013 | ) | (273,013 | ) | |||||||||||||||||||||||||||||||
| Net loss for the fiscal year ended June 30, 2017 | - | - | - | - | - | - | - | (7,867,500 | ) | - | - | (7,867,500 | ) | |||||||||||||||||||||||||||||||
| Balance at June 30, 2017 | 500,000 | $ | 5,000 | 1 | $ | - | 4,578,284 | $ | 4,578 | $ | 32,980,420 | $ | (38,243,523 | ) | $ | (46,477 | ) | $ | (141,749 | ) | $ | (5,441,751 | ) | |||||||||||||||||||||
| Issuance of common stock for conversion of convertible debt and accrued interest | - | - | - | - | 40,897,389 | 40,897 | 2,729,291 | - | - | - | 2,770,188 | |||||||||||||||||||||||||||||||||
| Reclassification of premium upon debt conversion | - | - | - | - | - | - | 948,129 | - | - | - | 948,129 | |||||||||||||||||||||||||||||||||
| Extinguishment of derivative liability associated with convertible notes | - | - | - | - | - | - | 809,642 | - | - | - | 809,642 | |||||||||||||||||||||||||||||||||
| Loss on settlement of debt, net | - | - | - | - | (15,000 | ) | (15 | ) | 55,216 | - | - | - | 55,201 | |||||||||||||||||||||||||||||||
| Issuance of stock for services | - | - | - | - | 968,750 | 969 | 129,031 | - | - | - | 130,000 | |||||||||||||||||||||||||||||||||
| Stock option expense | - | - | - | - | - | - | 516,148 | - | - | - | 516,148 | |||||||||||||||||||||||||||||||||
| Foreign currency translation loss | - | - | - | - | - | - | - | - | - | 499,678 | 499,678 | |||||||||||||||||||||||||||||||||
| Net loss for the fiscal year ended June 30, 2018 | - | - | - | - | - | - | - | (7,039,155 | ) | - | - | (7,039,155 | ) | |||||||||||||||||||||||||||||||
| Balance at June 30, 2018 | 500,000 | $ | 5,000 | 1 | $ | - | 46,429,423 | $ | 46,429 | $ | 38,167,877 | $ | (45,282,678 | ) | $ | (46,477 | ) | $ | 357,929 | $ | (6,751,920 | ) | ||||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-38 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Years Ended June 30, | ||||||||
| 2018 | 2017 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (7,039,155 | ) | $ | (7,867,500 | ) | ||
| Adjustments to Reconcile Net Loss to Net Cash Used in Operating Activities: | ||||||||
| Issuance and amortization of common stock for services | 130,000 | 670,037 | ||||||
| Issuance of convertible promissory notes for services | 310,000 | 500,000 | ||||||
| Warrant modification expense | - | 23,495 | ||||||
| Loss on settlements, net | 18,585 | 195,650 | ||||||
| Foreign currency transaction loss (gain) | 694,614 | (144,605 | ) | |||||
| Depreciation expense | 2,225 | 2,166 | ||||||
| Amortization of debt discount | 853,459 | 1,969,514 | ||||||
| Change in fair value of derivative liabilities | 7,612 | (820,153 | ) | |||||
| Gain on extinguishment of debt | (251,392 | ) | - | |||||
| Stock option expense | 516,148 | 1,686,444 | ||||||
| Reduction of put premium due to payment of debt | (80,769 | ) | - | |||||
| Accretion of put premium | 1,784,231 | 1,109,167 | ||||||
| Changes in Assets and Liabilities: | ||||||||
| GST receivable | 1,636 | 21,951 | ||||||
| Prepaid expenses and other assets | (29,842 | ) | (4,739 | ) | ||||
| Prepaid expenses and other assets - related parties | - | 2,263 | ||||||
| Accounts payable | 732,794 | 147,951 | ||||||
| Employee benefit liability | 28,052 | 23,538 | ||||||
| Payment for security deposit | - | 1,660 | ||||||
| Accrued expenses | (70,537 | ) | 331,660 | |||||
| Accrued interest | 214,694 | 100,865 | ||||||
| NET CASH USED IN OPERATING ACTIVITIES | (2,177,645 | ) | (2,050,636 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Loan repayments | (2,326 | ) | - | |||||
| Proceeds from convertible promissory notes | 2,890,080 | 1,634,500 | ||||||
| Repayments of convertible promissory notes | (490,181 | ) | - | |||||
| Proceeds from note payable | - | 20,000 | ||||||
| Repayment of note payable | - | (20,000 | ) | |||||
| Repayment of related party loans | (1,085 | ) | - | |||||
| Proceeds from the exercise of warrants | - | 464,286 | ||||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 2,396,488 | 2,098,786 | ||||||
| Effect of exchange rate changes on cash | (267,965 | ) | (100,177 | ) | ||||
| NET DECREASE IN CASH | (49,122 | ) | (52,027 | ) | ||||
| CASH AT BEGINNING OF YEAR | 69,043 | 121,070 | ||||||
| CASH AT END OF YEAR | $ | 19,921 | $ | 69,043 | ||||
| Supplemental Disclosure of Cash Flow Information | ||||||||
| Cash paid during the year: | ||||||||
| Interest | $ | 16,899 | $ | 537 | ||||
| Income Tax | $ | - | $ | - | ||||
| Supplemental Disclosure of Non-Cash Investing and Financing Activities | ||||||||
| Treasury stock re-purchased for reversal of debt conversion | $ | - | $ | 46,477 | ||||
| Cancellation of shares for convertible note payable | $ | - | $ | 112,500 | ||||
| Reduction of put premium related to conversions of convertible note | $ | 948,129 | $ | 266,287 | ||||
| Conversion of convertible notes and accrued interest to common stock | $ | 2,770,187 | $ | 1,406,736 | ||||
| Discounts related to warrants issued with convertible debenture | $ | - | $ | 910,178 | ||||
| Discounts related to derivative liability | $ | 543,744 | $ | 650,000 | ||||
| Settlement of accounts payable for shares of common stock | $ | - | $ | 50,000 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-39 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
NOTE 1 – NATURE OF OPERATIONS AND SUMMARY OF SIGNIFICANT ACCOUNTING AND REPORTING POLICIES
Nature of Operations
Propanc Biopharma, Inc. (the “Company,” “we,” “us” or “our” ) was originally incorporated in Melbourne, Victoria Australia on October 15, 2007 as Propanc PTY LTD, and continues to be based in Camberwell, Victoria Australia. Since its inception, substantially all of the operations of the Company have been focused on the development of new cancer treatments targeting high-risk patients, particularly cancer survivors, who need a follow-up, non-toxic, long-term therapy designed to prevent the cancer from returning and spreading. The Company anticipates establishing global markets for its technologies. Our lead product candidate, which we refer to as PRP, is an enhanced pro-enzyme formulation designed to enhance the anti-cancer effects of multiple enzymes acting synergistically. It is currently in the preclinical phase of development.
On November 23, 2010, the Company was incorporated in the state of Delaware as Propanc Health Group Corporation. In January 2011, to reorganize the Company, we acquired all of the outstanding shares of Propanc PTY LTD on a one-for-one basis making it a wholly-owned subsidiary of the Company.
On July 22, 2016, the Company formed a wholly owned subsidiary, Propanc (UK) Limited under the laws of England and Wales for the purpose of submitting an orphan drug application to the European Medicines Agency as a small and medium-sized enterprise. As of June 30, 2018, there has been no activity within this entity.
Effective April 20, 2017, the Company changed its name to “Propanc Biopharma, Inc.” to better reflect the Company’s stage of operations and development.
The Company has filed six patent applications relating to its lead product, PRP. The first application was filed in October 2010 in each of the countries listed in the table below. This application has been granted and remains in force in the United States, Europe, Australia, China, Japan, Indonesia, Israel, New Zealand, Singapore, Malaysia, South Africa, and Mexico. In Brazil, Canada, Hong Kong, and Republic of Korea, the patent application remains under examination.
In 2016 and 2017 we filed other patent applications, as indicated below. Three applications were filed under the Patent Cooperation Treaty (the “PCT”). The PCT assists applicants in seeking patent protection by filing one international patent application under the PCT, which allows the applicants to seek protection for an invention in over 150 countries. Once national or regional applications are filed, the application is placed under the control of the national or regional patent offices, as applicable, in what is called the national or regional phase. One PCT application, filed in November 2016, entered the national phase in July 2018 in each of the countries listed in the table below. A second application filed in January 2017 is currently entering the national phase commencing August, 2018 and a third application is scheduled to enter the national phase in October, 2018.
| F-40 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
| No. | Title | Country | Case Status | Date Filed | ||||
| 1. | A pharmaceutical composition for treating cancer comprising trypsinogen and/or chymotrypsinogen and an active agent selected from a selenium compound, a vanilloid compound and a cytoplasmic reduction agent. | USA, Europe, Australia, China, Japan, Indonesia, Israel, New Zealand, Malaysia, Singapore, South Africa and Mexico | Granted | Oct-22-2010 | ||||
| Brazil, Canada, Hong Kong, India and Republic of Korea | Under Examination | |||||||
| 2. | Proenzyme composition | Australia, Canada, China, Europe, India, Indonesia, Israel, Japan, Malaysia, New Zealand, Singapore, South Africa and USA | Application filed and pending | Nov-11-2016 | ||||
| 3. | Cancer Treatment | PCT | Application filed and pending | Jan-27-2017 | ||||
| 4. | Composition of proenzymes for cancer treatment | PCT | Application filed and pending | Apr-12-2017 |
The Company hopes to capture and protect additional patentable subject matter based on the Company’s field of technology relating to pharmaceutical compositions of proenzymes for treating cancer by filing additional patent applications as it advances its lead product candidate, PRP, through various stages of development.
Reverse Stock Split and Increase in Authorized Common Stock
On April 20, 2017, the Company effected a one-for-two hundred and fifty (1:250) reverse stock split whereby the Company (i) decreased the number of authorized shares of common stock, $0.001 par value per share, to 100,000,000 (ii) decreased the number of authorized shares of preferred stock, $0.01 par value per share to 1,500,005, and (iii) decreased by a ratio of one-for-two hundred and fifty (1:250) the number of retroactively issued and outstanding shares of common stock. Proportional adjustments for the reverse stock split were made to the Company’s outstanding stock options, warrants and equity incentive plans. All share and per-share data and amounts have been restated as of the earliest period, presented in the consolidated financial statements to reflect the reverse stock split.
On January 23, 2018, Company filed a Certificate of Amendment to its Certificate of Incorporation with the Secretary of State of the State of Delaware to increase the number of authorized shares of the Company’s common stock from 100,000,000 to 400,000,000.
Principles of Consolidation
The consolidated financial statements include the accounts of Propanc Biopharma, Inc., the parent entity, and its wholly-owned subsidiary, Propanc PTY LTD. All inter-company balances and transactions have been eliminated in consolidation. Propanc (UK) Limited was an inactive subsidiary at June 30, 2018.
Use of Estimates
The preparation of financial statements in conformity with the accounting principles generally accepted in the United States of America (“US GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates. Significant estimates in the accompanying consolidated financial statements include the estimates of useful lives for depreciation, valuation of derivatives, valuation of beneficial conversion features on convertible debt, allowance for uncollectable receivables, valuation of equity based instruments issued for other than cash, the valuation allowance on deferred tax assets and foreign currency translation due to certain average exchange rates applied in lieu of spot rates on transaction dates.
| F-41 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
Foreign Currency Translation and Other Comprehensive Income (Loss)
The Company’s functional currency is the Australian dollar (AUD). For financial reporting purposes, the Australian dollar has been translated into United States dollars ($) and/or (USD) as the reporting currency. Assets and liabilities are translated at the exchange rate in effect at the balance sheet date. Revenues and expenses are translated at the average rate of exchange prevailing during the reporting period. Equity transactions are translated at each historical transaction date spot rate. Translation adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’ equity (deficit) as “Accumulated other comprehensive income (loss).” Gains and losses resulting from foreign currency transactions are included in the statements of operations and comprehensive income (loss) as other comprehensive income (loss). There have been no significant fluctuations in the exchange rate for the conversion of Australian dollars to USD after the balance sheet date.
Other Comprehensive Income (Loss) for all periods presented includes only foreign currency translation gains (losses).
Assets and liabilities denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing at the consolidated balance sheet date with any transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency included in the consolidated results of operations as incurred.
As of June 30, 2018 and June 30, 2017, the exchange rates used to translate amounts in Australian dollars into USD for the purposes of preparing the consolidated financial statements were as follows:
| June 30, 2018 | June 30, 2017 | |||||||
| Exchange rate on balance sheet dates | ||||||||
| USD : AUD exchange rate | 0.7399 | 0.7676 | ||||||
| Average exchange rate for the period | ||||||||
| USD : AUD exchange rate | 0.7753 | 0.7544 | ||||||
Changes in Accumulated Other Comprehensive Income (Loss) by component during the years ended June 30, 2018 and 2017 were as follows:
| Foreign Currency Items: | ||||
| Beginning balance, June 30, 2016 | $ | 131,264 | ||
| Foreign currency translation gain | (273,013 | ) | ||
| Balance, June 30, 2017 | (141,749 | ) | ||
| Foreign currency translation loss | 499,678 | |||
| Ending balance, June 30, 2018 | $ | 357,929 | ||
Fair Value of Financial Instruments and Fair Value Measurements
The Company measures its financial assets and liabilities in accordance with US GAAP. For certain financial instruments, including cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities, the carrying amounts approximate fair value due to their short maturities. Amounts recorded for notes payable, net of discount, and loans payable also approximate fair value because current interest rates available for debt with similar terms and maturities are substantially the same.
The Company follows accounting guidance for financial assets and liabilities. This standard defines fair value, provides guidance for measuring fair value and requires certain disclosures. This standard does not require any new fair value measurements, but rather applies to all other accounting pronouncements that require or permit fair value measurements. This guidance does not apply to measurements related to share-based payments. This guidance discusses valuation techniques, such as the market approach (comparable market prices), the income approach (present value of future income or cash flow), and the cost approach (cost to replace the service capacity of an asset or replacement cost).
| F-42 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
The guidance utilizes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The following is a brief description of those three levels:
Level 1: Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2: Inputs, other than quoted prices that are observable, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active.
Level 3: Unobservable inputs in which little or no market data exists, therefore developed using estimates and assumptions developed by us, which reflect those that a market participant would use.
Also see Note 12 - Derivative Financial Instruments and Fair Value Measurements.
Cash and Cash Equivalents
Cash and cash equivalents include cash on hand and at banks, short-term deposits with an original maturity of three months or less with financial institutions, and bank overdrafts. Bank overdrafts are reflected as a current liability on the balance sheets. There were no cash equivalents as of June 30, 2018 or June 30, 2017.
Receivables
As amounts become uncollectible, they will be charged to an allowance and operations in the period when a determination of uncollectability is made. Any estimates of potentially uncollectible customer accounts receivable will be made based on an analysis of individual customer and historical write-off experience. The Company’s analysis includes the age of the receivable account, creditworthiness of the customer and general economic conditions.
Property and Equipment
Property and equipment are stated at cost, net of accumulated depreciation. Expenditures for maintenance and repairs are expensed as incurred; additions, renewals, and betterments are capitalized. When property and equipment are retired or otherwise disposed of, the related cost and accumulated depreciation are removed from the respective accounts, and any gain or loss is included in operations. Depreciation of property and equipment is provided using the declining balance method. The depreciable amount is the cost less its residual value.
The estimated useful lives are as follows:
| Machinery and equipment | - 5 years |
| Furniture | - 7 years |
Patents
Patents are stated at cost and reclassified to intangible assets and amortized on a straight-line basis over the estimated future periods if and once the patent has been granted by a regulatory agency. However, the Company will expense any product costs as long as we are in the startup stage. Accordingly, as the Company’s products were and are not currently approved for market, all patent costs incurred from 2013 through 2018 were expensed immediately. This practice of expensing patent costs immediately ends when a product receives market authorization from a government regulatory agency.
Impairment of Long-Lived Assets
In accordance with ASC 360-10, “Long-lived assets,” which include property and equipment and intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of long-lived assets to be held and used is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the assets. Fair value is generally determined using the asset’s expected future discounted cash flows or market value, if readily determinable.
| F-43 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
Employee Benefit/Liability
Liabilities arising in respect of wages and salaries, annual leave, accumulated sick leave and any other employee benefits expected to be settled within twelve months of the reporting date are measured at their nominal amounts based on remuneration rates which are expected to be paid when the liability is settled. All other employee benefit liabilities are measured at the present value of the estimated future cash outflow to be made in respect of services provided by employees up to the reporting date. All employee liabilities are owed within the next twelve months and therefore, recorded at nominal value.
Australian Goods and Services Tax (“GST”)
Revenues, expenses and balance sheet items are recognized net of the amount of GST, except payable and receivable balances which are shown inclusive of GST. The GST incurred is payable on revenues to, and recoverable on purchases from, the Australian Taxation Office.
Cash flows are presented in the statements of cash flow on a gross basis, except for the GST component of investing and financing activities, which are disclosed as operating cash flows.
As of June 30, 2018 and June 30, 2017, the Company was owed $6,257 and $8,111, respectively, from the Australian Taxation Office. These amounts were fully collected subsequent to the balance sheet reporting dates.
Derivative Instruments
ASC Topic 815, Derivatives and Hedging (“ASC Topic 815”), establishes accounting and reporting standards for derivative instruments and for hedging activities by requiring that all derivatives be recognized in the balance sheet and measured at fair value. Gains or losses resulting from changes in the fair value of derivatives are recognized in earnings. On the date of conversion or payoff of debt, the Company records the fair value of the conversion shares, removes the fair value of the related derivative liability, removes any discounts and records a net gain or loss on debt extinguishment.
Convertible Notes With Variable Conversion Options
The Company has entered into convertible notes, some of which contain variable conversion options, whereby the outstanding principal and accrued interest may be converted, by the holder, into common shares at a fixed discount to the price of the common stock at the time of conversion. The Company treats these convertible notes as stock settled debt under ASC 480, “Distinguishing Liabilities from Equity” and measures the fair value of the notes at the time of issuance, which is the result of the share price discount at the time of conversion and records the put premium as accretion to interest expense to the date of first conversion.
Income Taxes
The Company is governed by Australia and United States income tax laws, which are administered by the Australian Taxation Office and the United States Internal Revenue Service, respectively. The Company follows ASC 740 “Accounting for Income Taxes,” when accounting for income taxes, which requires an asset and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed annually for temporary differences between the financial statements and tax bases of assets and liabilities that will result in taxable or deductible amounts in the future based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Income tax expense is the tax payable or refundable for the period plus or minus the change during the period in deferred tax assets and liabilities.
| F-44 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
The Company follows ASC 740, Sections 25 through 60, “Accounting for Uncertainty in Income Taxes.” These sections provide detailed guidance for the financial statement recognition, measurement and disclosure of uncertain tax positions recognized in the financial statements. Tax positions must meet a “more-likely-than-not” recognition threshold at the effective date to be recognized upon the adoption of ASC 740 and in subsequent periods.
On December 22, 2017, the passage of legislation commonly referred to as the Tax Cuts and Jobs Act (“TCJA”) was enacted and significantly revised the U.S. income tax law. The TCJA includes changes, which reduce the corporate income tax rate from 34% to 21% for fiscal years beginning after December 31, 2017. On December 22, 2017, the SEC Staff Accounting Bulletin No. 118 (“SAB 118”) was issued, which allows a company to recognize provisional tax amounts when it does not have the necessary information available, prepared or analyzed, including computations, in reasonable detail to complete its accounting for the change in tax law. SAB 118 provides for a measurement of up to one year from the date of enactment.
Research and Development Costs and Tax Credits
In accordance with ASC 730-10, “Research and Development-Overall,” research and development costs are expensed when incurred. Total research and development costs for the fiscal years ended June 30, 2018 and 2017 were $1,825,728 and $971,769, respectively.
The Company may apply for research and development tax concessions with the Australian Taxation Office on an annual basis. Although the amount is possible to estimate at year end, the Australian Taxation Office may reject or materially alter the claim amount. Accordingly, the Company does not recognize the benefit of the claim amount until cash receipt since collectability is not certain until such time. The tax concession is a refundable credit. If the Company has net income then the Company can receive the credit which reduces its income tax liability. If the Company has net losses, then the Company may still receive a cash payment for the credit, however, the Company’s net operating loss carryforwards are reduced by the gross equivalent loss that would produce the credit amount when the income tax rate is applied to that gross amount. The concession is recognized as a tax benefit, in operations, upon receipt.
During the fiscal years ended June 30, 2018 and 2017, the Company applied for and received from the Australian Taxation Office a research and development tax credit in the amount of $179,306 and $305,673 respectively, which is reflected as a tax benefit in the Company’s accompanying consolidated statements of operations and comprehensive income (loss).
Stock Based Compensation
The Company records stock-based compensation in accordance with ASC 718, “Stock Compensation” and the SEC Staff Accounting Bulletin No. 107 Share Based Payment was issued in March 2005 regarding its interpretation of ASC 718. ASC 718 requires the fair value of all stock-based employee compensation awarded to employees to be recorded as an expense over the related requisite service period. The Company values employee and non-employee stock-based compensation at fair value using the Black-Scholes Option Pricing Model.
The Company accounts for non-employee share-based awards in accordance with the measurement and recognition criteria of ASC 505-50 “Equity-Based Payments to Non-Employees.”
Revenue Recognition
In accordance with ASC 605, the Company intends to recognize revenue when (i) persuasive evidence of a customer or distributor arrangement exists or acceptance occurs, (ii) a retailer, distributor or wholesaler receives the goods, (iii) the price is fixed or determinable, and (iv) collectability of the sales revenues is reasonably assured. Subject to these criteria, the Company intends to recognize revenue relating to royalties on product sales in the period in which the sale occurs and the royalty term has begun.
Legal Expenses
All legal costs for litigation are charged to expense as incurred.
| F-45 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
Basic and Diluted Net Loss Per Common Share
Basic net loss per share is computed by dividing the net loss by the weighted average number of common shares outstanding during the period. Diluted net loss per common share is computed by dividing the net loss by the weighted average number of common shares outstanding for the period and, if dilutive, potential common shares outstanding during the period. Potentially dilutive securities consist of the incremental common shares issuable upon exercise of common stock equivalents such as stock options, warrants and convertible debt instruments. Potentially dilutive securities are excluded from the computation if their effect is anti-dilutive. As a result, the basic and diluted per share amounts for all periods presented are identical. For the years ended June 30, 2018 and 2017, there were 145,517 and 149,517 warrants, respectively, outstanding, 572,000 and 572,000 stock options outstanding, respectively, and 20 and 13 convertible notes payable, respectively, which notes are convertible into 103,698,414 and 4,388,155 shares of the Company’s common stock, respectively, which are considered dilutive securities which were excluded from the computation since the effect is anti-dilutive.
Recently Adopted Accounting Pronouncements
ASU 2018-07 - In June 2018, the Financial Accounting Standards Board (the “FASB”) issued Accounting Standards Update (“ASU”) 2018-07, Compensation – Stock Compensation (Topic 718). This update is intended to reduce cost and complexity and to improve financial reporting for share-based payments issued to non-employees (for example, service providers, external legal counsel, suppliers, etc.). The ASU expands the scope of Topic 718, Compensation—Stock Compensation, which currently only includes share-based payments issued to employees, to also include share-based payments issued to non-employees for goods and services. Consequently, the accounting for share-based payments to non-employees and employees will be substantially aligned. This standard will be effective for financial statements issued by public companies for the annual and interim periods beginning after December 15, 2018. Early adoption of the standard is permitted. The standard will be applied in a retrospective approach for each period presented. Management currently does not plan to early adopt this guidance and is evaluating the potential impact of this guidance on the Company’s consolidated financial statements as well as transition methods.
ASU 2017-01 - In January 2017, the FASB issued ASU No. 2017-01: “Business Combinations (Topic 805)- to clarify the definition of a business with the objective of adding guidance to assist entities with evaluating whether transactions should be accounted for as acquisitions (or disposals) of assets or businesses. This guidance is effective for interim and annual reporting periods beginning after December 15, 2017. The Company implemented this guidance effective January 1, 2018.
ASU No 2016-18 – In November 2016, the FASB issue ASU No. 2016-18, Statement of Cash Flows (Topic 230) Restricted Cash (ASU 2016-18), requiring restricted cash and cash equivalents to be included with cash and cash equivalents of the statement of cash flows. The new standard is effective for fiscal years, and interim periods with those year, beginning December 15, 2017, with early adoption permitted. The Company has elected to adopt this new ASU at July 1, 2018 and does not anticipate the ASU to have a material impact on its consolidated financial statements.
ASU 2016-02 - In February 2016, the FASB issued ASU No. 2016-02: “Leases (Topic 842)” whereby lessees will need to recognize almost all leases on their balance sheet as a right of use asset and a lease liability. This guidance is effective for interim and annual reporting periods beginning after December 15, 2018. The Company does not anticipate the ASU to have a material impact on its consolidated financial statements.
ASU 2014-09 - In May 2014, the FASB issued ASU No. 2014-09: “Revenue from Contracts with Customers (Topic 606)” which requires that an entity recognize revenue to depict the transfer of promised goods and services to customers in an amount that reflects the consideration to which the Company expects to be entitled in exchange for those goods or services. Since the issuance of the original standard, the FASB has issued several updates to the standard which (i) clarify the application of the principal versus agent guidance; (ii) clarify the guidance relating to performance obligations and licensing; (iii) clarify assessment of the collectability criterion, presentation of sales taxes, measurement date for non-cash consideration and completed contracts at transaction; and (iv) clarify narrow aspects of ASC 606 or corrects unintended application of the guidance. The new revenue recognition standard, amended by the updates, becomes effective in the first quarter of fiscal 2019 and is to be applied retrospectively using one of two prescribed methods. Early adoption is permitted. The Company adopted the new standard effective July 1, 2018.
| F-46 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
NOTE 2 – GOING CONCERN
The accompanying consolidated financial statements have been prepared in conformity with US GAAP, which contemplate continuation of the Company as a going concern. For the year ended June 30, 2018, the Company had no revenues, had a net loss of $7,039,155 and had net cash used in operations of $2,177,645. Additionally, as of June 30, 2018, the Company had a working capital deficit, stockholders’ deficit and accumulated deficit of $6,762,417, $6,751,920 and $45,282,678, respectively. It is management’s opinion that these conditions raise substantial doubt about the Company’s ability to continue as a going concern for a period of twelve months from the date of this filing.
The consolidated financial statements do not include any adjustments to reflect the possible future effect on the recoverability and classification of assets or the amounts and classifications of liabilities that may result from the outcome of this uncertainty.
Successful completion of the Company’s development program and, ultimately, the attainment of profitable operations are dependent upon future events, including obtaining adequate financing to fulfill its development activities, acceptance of the Company’s patent applications obtaining additional sources of suitable and adequate financing and ultimately achieving a level of sales adequate to support the Company’s cost structure. The Company’s ability to continue as a going concern is also dependent on its ability to further develop and execute on its business plan. However, there can be no assurances that any or all of these endeavors will be successful.
NOTE 3 – PROPERTY AND EQUIPMENT
Property and equipment consist of the following as of June 30,
| 2018 | 2017 | |||||||
| Office equipment at cost | $ | 25,244 | $ | 26,189 | ||||
| Less: Accumulated depreciation | (16,967 | ) | (15,399 | ) | ||||
| Total property, plant, and equipment | $ | 8,277 | $ | 10,790 | ||||
Depreciation expense for the years ended June 30, 2018 and 2017 were $2,225 and $2,166, respectively.
NOTE 4 – DUE TO FORMER DIRECTORS - RELATED PARTIES
Due to directors - related parties represents unsecured advances made primarily by a former director for operating expenses on behalf of the Company such as intellectual property and formation expenses. The expenses were paid for on behalf of the Company and are due upon demand. The Company is currently not being charged interest under these advances. The total amount owed the former director at June 30, 2018 and June 30, 2017 is $32,898 and $35,204, respectively. The Company plans to repay the notes as its cash resources allow.
NOTE 5 – LOANS AND NOTES PAYABLE
Loans from Directors and Officer - Related Parties
Loans from Directors and Officer at June 30, 2018 and June 30, 2017 were $54,753 and $56,802, respectively. The loans bear no interest and are all payable on demand. The Company did not repay any amount on these loans during the years ended June 30, 2018 and 2017. The Company plans to repay the notes as its cash resources allow.
| F-47 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
Other Loans from Unrelated Parties
As of June 30, 2018 and June 30, 2017, other loans from unrelated parties had a balance of $0 and $2,303, respectively. The Company repaid these loans in full during the year ended June 30, 2018.
NOTE 6 – CONVERTIBLE NOTES
Convertible notes at June 30, 2018 and 2017 were as follows:
| June 30, 2018 | June 30, 2017 | |||||||
| Convertible notes and debenture | $ | 3,096,935 | $ | 2,863,271 | ||||
| Unamortized discounts | (277,733 | ) | (445,594 | ) | ||||
| Accrued interest | 148,930 | 86,334 | ||||||
| Premium, net | 1,731,167 | 975,834 | ||||||
| Convertible notes, net | $ | 4,699,299 | $ | 3,479,845 | ||||
May 2015 Securities Purchase Agreement
On May 19, 2015, the Company entered into a Securities Purchase Agreement with a third-party lender (the “SPA”). Pursuant to the SPA, on the date of the agreement the Company issued convertible promissory notes to the lender in return for cash. The Company also issued nine convertible promissory notes in the principal amount of $782,500 (the “Back-End Notes”) in exchange for promissory notes from the lender in the same principal amount. The lender could not convert the Back-End Notes until it had redeemed its notes for cash.
On July 14, 2015, the lender redeemed three of its promissory notes totaling $352,500 and three of the Back-End Notes of the same principal amount it received from the Company automatically became convertible.
On October 14 and October 15, 2015, the lender redeemed the remaining six of its promissory notes totaling $430,000 and the corresponding six remaining Back-End Notes of the same principal amount became convertible.
Through June 30, 2016, the lender converted $620,000 in principal of the Back-End Notes into an equivalent amount of shares of the Company’s common stock. From June 30, 2016 through June 30, 2017, the remaining $162,500 in principal of the Back-End notes along with $15,091 in accrued interest was converted into 137,348 shares of the Company’s common stock (See Note 8 – Stockholders’ Deficit). As of June 30, 2017, these Back-End Notes have been fully converted.
Delafield Financing Agreements
Initial Securities Purchase Agreement and Debenture
On October 28, 2015, the Company entered into a securities purchase agreement with Delafield Investments Limited (the “Purchaser” or “Delafield”), whereby the Purchaser purchased a $4,000,000 5% convertible debenture in the principal amount of $4,350,000 from the Company. Additionally, Delafield received 4-year warrants to purchase an aggregate of 104,762 shares of the Company’s common stock with an exercise price of $3.00 per share. As of June 30, 2017, the principal balance of the convertible debenture was $720,271 and the related derivative liability associated with the convertible debenture was $252,303. During the year ended June 30, 2018, the Company converted $380,090 in principal and $8,250 in accrued interest under the debenture into shares of the Company’s common stock (see Note 8 – Stockholders’ Deficit). On January 2, 2018, the Company repaid the remaining principal balance of $340,181, the derivative liability was revalued, and the Company recorded $199,339 to gain on debt extinguishment.
Additional Debenture
On September 13, 2016, the Company entered into an Additional Issuance Agreement (“Additional Debenture”) with the Purchaser whereby the Purchaser loaned an additional $150,000 to the Company in exchange for a 5% Original Issue Discount Senior Secured Convertible Debenture of the Company in the principal amount of $165,000. As of June 30, 2017, the Company recorded accrued interest of $8,250 and had a principal balance of $165,000 outstanding under the Additional Debenture. Additional at June 30, 2017, the derivative liability related to the Additional Debenture was $54,727. At June 30, 2018, all $165,000 in outstanding principal under the Additional Debenture along with $8,250 of accrued interest was fully converted into shares of the Company’s common stock (see Note 8 – Stockholders’ Deficit).
| F-48 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
December 2016 Letter Agreement
On December 2, 2016, the Company entered into a Letter Agreement with the Purchaser pursuant to which the parties agreed to cancel the warrants to purchase up to 960,000 shares of the Company’s common stock issued to the Purchaser as of such date in exchange for an 8% convertible redeemable promissory note in the principal amount of $150,000. As of June 30, 2017, the Company recorded accrued interest of $6,937 and had a principal balance of $150,000 outstanding. On January 2, 2018, the Company repaid the remaining principal balance of $150,000 and accrued interest of $16,899. In connection with the Letter Agreement, the Company issued the Purchaser a 2-year common stock purchase warrants to purchase 104,000 shares of Company’s common stock at an exercise price of $12.50 per share.
Eagle Equities Finance Agreements
October 31, 2016 Securities Purchase Agreement
On October 31, 2016, the Company entered into a Securities Purchase Agreement with Eagle Equities, LLC (“Eagle Equities”), pursuant to which Eagle Equities purchased two 8% convertible redeemable junior subordinated promissory notes, each in the principal amount of $100,000. The first note (the “October 2016 Eagle Note”) was funded with cash and the second note (the “October 2016 Eagle Back-End Note”) was initially paid for by an offsetting promissory note issued by Eagle Equities to the Company (the “October 2016 Eagle Note Receivable”). The terms of the October 2016 Eagle Back-End Note require cash funding prior to any conversion thereunder. The October 2016 Eagle Note Receivable was due June 30, 2017, unless certain conditions were not met, in which case both the October 2016 Eagle Back-End Note and the October 2016 Eagle Note Receivable may have both been cancelled. Both the October 2016 Eagle Note and the October 2016 Eagle Back-End Note had a maturity date one year from the date of issuance upon which any outstanding principal and interest was due and payable. The amounts cash funded plus accrued interest under both the October 2016 Eagle Note and the October 2016 Eagle Back-End Note are convertible into common stock at a conversion price equal to 60% of the lowest closing bid price of the Company’s common stock for the ten trading days prior to the conversion, subject to adjustment in certain events. On April 11, 2017, the Company received payment of the October 2016 Eagle Note Receivable in the amount of $100,000 that offset the October 2016 Eagle Back-End Note. Proceeds from the October 2016 Eagle Note Receivable of $5,000 were paid directly to legal fees resulting in net cash proceeds of $95,000 received by the Company. As a result, the October 2016 Eagle Back-End Note is now convertible. The October 2016 Eagle Note and the October 2016 Eagle Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company recorded a put premium of $66,667 as each of the notes were funded. As of June 30, 2017, the outstanding principal under the October 2016 Eagle Note and the October 2016 Eagle Back-End Note along with $4,509 and $1,732, respectively, of accrued interest was fully converted (see Note 8 – Stockholders’ Deficit) and the repayments resulted in a full reduction of the put premiums.
December 12, 2016 Securities Purchase Agreement
On December 12, 2016, the Company entered into a Securities Purchase Agreement, with Eagle Equities, pursuant to which Eagle Equities purchased from the Company two 8% convertible redeemable junior subordinated promissory notes, each in the principal amount of $100,000. The first note (the “December 12 Eagle Note”) was funded with cash and the second note (the “December 12 Eagle Back-End Note”) was initially paid for by an offsetting promissory note issued by Eagle Equities to the Company (the “December 12 Eagle Note Receivable”). The terms of the December 12 Eagle Back-End Note require cash funding prior to any conversion thereunder. The December 12 Eagle Note Receivable was due December 12, 2017, unless certain conditions were not met, in which case both the December 12 Eagle Back-End Note and the December 12 Eagle Note Receivable may have both been cancelled. Both the December 12 Eagle Note and the December 12 Eagle Back-End Note had a maturity date one year from the date of issuance upon which any outstanding principal and interest is due and payable. The outstanding principal amounts plus accrued interest under both the December 12 Eagle Note and the December 12 Eagle Back-End Note are convertible into the Company’s common stock at a conversion price equal to 60% of the lowest closing bid price of the common stock for the ten trading days prior to the conversion, subject to adjustment in certain events. On April 11, 2017, the Company received payment of the December 12 Eagle Note Receivable in the amount of $100,000 that offset the December 12 Eagle Back-End Note. Proceeds from the December 12 Eagle Note Receivable of $5,000 were paid directly to legal fees resulting in net cash proceeds of $95,000 received by the Company. As a result, the December 12 Eagle Back-End Note is now convertible. The December 12 Eagle Note and the December 12 Eagle Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company recorded a put premium of $66,667 as each of the notes were funded. As of June 30, 2018, the outstanding principal under the December 12 Eagle Note along with $8,296 of accrued interest was fully converted into shares of the Company’s common stock (see Note 8 – Stockholders’ Deficit) and the repayment resulted in a full reduction of the put premium. The Company has recorded $9,775 of accrued interest on the December 12 Eagle Back-End Note as of June 30, 2018 and total principal outstanding on the December 12 Eagle Back-End Note as of June 30, 2018, was $100,000. The December 12 Eagle Back-End Note matured on December 12, 2017. The Company is currently in discussions with Eagle Equities to extend the maturity date.
| F-49 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
The December 12 Eagle Back-End Note may not be prepaid by the Company.
Upon an event of default, principal and accrued interest will become immediately due and payable under the notes. Additionally, upon an event of default, both notes will accrue interest at a default interest rate of 24% per annum or the highest rate of interest permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
December 21, 2016 Securities Purchase Agreement
On December 21, 2016, the Company entered into a Securities Purchase Agreement with Eagle Equities pursuant to which Eagle Equities purchased two 8% convertible redeemable junior subordinated promissory notes, each in the principal amount of $157,500. The first note (the “December 21 Eagle Note”) was funded with cash and the second note (the “December 21 Eagle Back-End Note”) was initially paid for by an offsetting promissory note issued by Eagle Equities to the Company (the “December 21 Eagle Note Receivable”). The terms of the December 21 Eagle Back-End Note require cash funding prior to any conversion thereunder. The December 21 Eagle Note Receivable was due December 21, 2017, unless certain conditions were not met, in which case both the December 21 Eagle Back-End Note and the December 21 Eagle Note Receivable may have both been cancelled. Both the December 21 Eagle Note and the December 21 Eagle Back-End Note had a maturity date one year from the date of issuance upon which any outstanding principal and interest is due and payable. The outstanding principal amounts plus accrued interest under both the December 21 Eagle Note and the December 21 Eagle Back-End Note were convertible into shares of the Company’s common stock at a conversion price equal to 60% of the lowest closing bid price of the common stock for the ten trading days prior to the conversion, subject to adjustment in certain events. On May 4, 2017, the Company received payment of the December 21 Eagle Note Receivable in the amount of $157,500 that offset the December 21 Eagle Back-End Note. Proceeds from the December 21 Eagle Note Receivable of $7,500 were paid directly to legal fees resulting in net cash proceeds of $150,000 received by the Company. As a result, the December 21 Eagle Back-End Note then became convertible. The December 21 Eagle Note and the December 21 Eagle Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company recorded a put premium of $105,000 as each of the notes were funded. As of June 30, 2018, the outstanding principal under the December 21 Eagle Note and the December 21 Eagle Back-End Note along with $7,773 and $5,656, respectively, of accrued interest was fully converted (see Note 8 – Stockholders’ Deficit) and the repayments resulted in a full reduction of the put premiums.
January 27, 2017 Securities Purchase Agreement
On January 27, 2017, the Company entered into a Securities Purchase Agreement with Eagle Equities, pursuant to which Eagle Equities purchased two 8% convertible redeemable junior subordinated promissory notes, each in the principal amount of $230,000. The first note (the “January 2017 Eagle Note”) was funded with cash and the second note (the “January 2017 Eagle Back-End Note”) was initially paid for by an offsetting promissory note issued by Eagle Equities to the Company (the “January 2017 Eagle Note Receivable”). The terms of the January 2017 Eagle Back-End Note require cash funding prior to any conversion thereunder. The January 2017 Eagle Note Receivable was due September 27, 2017, unless certain conditions are not met, in which case both the January 2017 Eagle Back-End Note and the January 2017 Eagle Note Receivable may have both been cancelled. Both the January 2017 Eagle Note and the January 2017 Eagle Back-End Note have a maturity date one year from the date of issuance upon which any outstanding principal and interest is due and payable. The outstanding principal amounts plus accrued interest under both the January 2017 Eagle Note and the January 2017 Eagle Back-End Note are convertible into common stock of the Company at a conversion price equal to 60% of the lowest closing bid price of the common stock for the ten trading days prior to the conversion, subject to adjustment in certain events. On May 4, 2017, the Company received a partial payment of the January 2017 Note Receivable in the amount of $40,000 and on June 3, 2017 the balance of $190,000 was funded, of which $11,250 was paid directly to legal fees. As a result, the January 2017 Eagle Back-End Note then became convertible. The January 2017 Eagle Note and the January 2017 Eagle Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company is recording a put premium of $153,333 as each of the notes were funded. As of June 30, 2018, the outstanding principal under the January 2017 Eagle Note along with $14,988 of accrued interest was fully converted (see Note 8 – Stockholders’ Deficit) and the repayment resulted in a full reduction of the put premium. The Company has recorded $20,475 of accrued interest as of June 30, 2018 for the January 2017 Eagle Back-End and total principal outstanding under the January 2017 Eagle Back-End Note as of June 30, 2018 was $230,000. The January 2017 Eagle Back-End Note matured on January 27, 2018. The Company is currently in discussions with Eagle Equities to extend the maturity date.
| F-50 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
The January 2017 Eagle Back-End Note may not be prepaid by the Company.
Upon an event of default, principal and accrued interest will become immediately due and payable under the notes. Additionally, upon an event of default, both notes will accrue interest at a default interest rate of 24% per annum or the highest rate of interest permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
March 1, 2017 Securities Purchase Agreement
On March 1, 2017, the Company entered into a Securities Purchase Agreement with Eagle Equities, pursuant to which Eagle Equities purchased two 8% convertible redeemable junior subordinated promissory notes, each in the principal amount of $220,500. The first note (the “March 2017 Eagle Note”) was funded with cash and the second note (the “March 2017 Eagle Back-End Note”) was initially paid for by an offsetting promissory note issued by Eagle Equities to the Company (the “March 2017 Eagle Note Receivable”). The terms of the March 2017 Eagle Back-End Note require cash funding prior to any conversion thereunder. Both the March 2017 Eagle Note and the March 2017 Eagle Back-End Note had a maturity date of March 1, 2018, upon which any outstanding principal and interest was due and payable. The outstanding principal amounts plus accrued interest under both the March 2017 Eagle Note and the March 2017 Eagle Back-End Note are convertible into shares of common stock, of the Company at a conversion price equal to 60% of the lowest closing bid price of the common stock for the ten trading days prior to the conversion, subject to adjustment in certain events. On July 5, 2017, the Company received payment of the March 2017 Eagle Note Receivable in the amount of $220,500 that offset the March 2017 Eagle Back-End Note. Proceeds from the March 2017 Eagle Note Receivable of $10,500 were paid directly to legal fees resulting in net cash proceeds of $210,000 received by the Company. As a result, the March 2017 Eagle Back-End Note then became now convertible. The March 2017 Eagle Note and the March 2017 Eagle Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company recorded a put premium of $147,000 as each of the notes were funded. As of June 30, 2018, the outstanding principal balance under the March 2017 Eagle Note along with $20,061 of accrued interest was fully converted (see Note 8 – Stockholders’ Deficit) and the repayment resulting in a full reduction of the put premium. The Company has recorded $17,447 of accrued interest as of June 30, 2018 for the March 2017 Eagle Back-End Note and total principal outstanding as of June 30, 2018 under the March 2017 Eagle Back-End Note was $220,500. The March 2017 Eagle Back-End Note matured on March 1, 2018. As of the date of the filing of this Annual Report, the March 2017 Eagle Back-End Note and accrued interest of $18,625 has been fully converted.
August 9, 2017 Securities Purchase Agreement
On August 9, 2017, the Company entered into a Securities Purchase Agreement dated as of August 8, 2017, with Eagle Equities, pursuant to which Eagle Equities purchased two 8% convertible redeemable junior subordinated promissory notes, each in the principal amount of $200,000. The first note (the “August 2017 Eagle Note”) was funded with cash and the second note (the “August 2017 Eagle Back-End Note”) was initially paid for by an offsetting promissory note issued by Eagle Equities to the Company (the “August 2017 Eagle Note Receivable”). The terms of the August 2017 Eagle Back-End Note require cash funding prior to any conversion thereunder. The August 2017 Eagle Note Receivable is due August 8, 2018, unless certain conditions are not met, in which case both the August 2017 Eagle Back-End Note and the August 2017 Eagle Note Receivable may both be cancelled. Both the August 2017 Eagle Note and the August 2017 Eagle Back-End Note have a maturity date one year from the date of issuance upon which any outstanding principal and interest is due and payable. The outstanding principal amounts plus accrued interest under both the August 2017 Eagle Note and the August 2017 Eagle Back-End Note are convertible into common stock of the Company at a conversion price equal to 60% of the lowest closing bid price of the common stock for the ten trading days prior to the conversion, subject to adjustment in certain events. On September 14, 2017, the Company received payment of the August 2017 Eagle Note Receivable in the amount of $200,000 that offset the August 2017 Eagle Back-End Note. Proceeds from the August 2017 Eagle Note Receivable of $10,000 were paid directly to legal fees resulting in net cash proceeds of $190,000 received by the Company. As a result, the August 2017 Eagle Back-End Note is now convertible. The August 2017 Eagle Note and the August 2017 Eagle Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company recorded a put premium of $133,333 as each of the notes were funded. The Company has recorded $6,661 of accrued interest as of June 30, 2018 for the August 2017 Eagle Note and total principal outstanding as of June 30, 2018 under the August 2017 Eagle Note was $80,000 as $120,000 was converted during the year ended June 30, 2018 (see Note 8 – Stockholders’ Deficit). The Company has recorded $12,712 of accrued interest as of June 30, 2018 for the August 2017 Eagle Back-End Note and total principal outstanding as of June 30, 2018 under the August 2017 Eagle Back-End Note was $200,000.
| F-51 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
The August 2017 Eagle Back-End Note may not be prepaid by the Company.
Upon an event of default, principal and accrued interest will become immediately due and payable under the notes. Additionally, upon an event of default, both notes will accrue interest at a default interest rate of 24% per annum or the highest rate of interest permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
October 25, 2017 Securities Purchase Agreement
On November 3, 2017, the Company entered into a Securities Purchase Agreement dated as of October 25, 2017, with Eagle Equities, pursuant to which Eagle Equities purchased two 8% convertible redeemable junior subordinated promissory notes, each in the principal amount of $200,000. The first note (the “October 2017 Eagle Note”) was funded with cash and the second note (the “October 2017 Eagle Back-End Note”) was initially paid for by an offsetting promissory note issued by Eagle Equities to the Company (the “October 2017 Eagle Note Receivable”). The terms of the October 2017 Eagle Back-End Note require cash funding prior to any conversion thereunder. The October 2017 Eagle Note Receivable is due June 25, 2018, unless certain conditions are not met, in which case both the October 2017 Eagle Back-End Note and the October 2017 Eagle Note Receivable may both be cancelled. Both the October 2017 Eagle Note and the October 2017 Eagle Back-End Note have a maturity date one year from the date of issuance upon which any outstanding principal and interest is due and payable. The amounts cash funded plus accrued interest under both the October 2017 Eagle Note and the October 2017 Eagle Back-End Note are convertible into common stock, par value $0.001 of the Company at a conversion price equal to 60% of the lowest closing bid price of the common stock for the ten trading days prior to the conversion, subject to adjustment in certain events. On December 6, 2017, the Company received payment of the October 2017 Eagle Note Receivable in the amount of $200,000 that offset the October 2017 Eagle Back-End Note. Proceeds from the October 2017 Eagle Note Receivable of $10,000 were paid directly to legal fees resulting in net cash proceeds of $190,000 received by the Company. As a result, the October 2017 Eagle Back-End Note is now convertible. The October 2017 Eagle Note and the October 2017 Eagle Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company recorded a put premium of $133,333 as each of the notes were funded. The Company has recorded $10,608 of accrued interest as of June 30, 2018 for the October 2017 Eagle Note and total principal outstanding as of June 30, 2018 under the October 2017 Eagle Note was $200,000. The Company has recorded $9,074 of accrued interest as of June 30, 2018 for the October 2017 Eagle Back-End Note and total principal outstanding as of June 30, 2018 under the October 2017 Eagle Back-End Note was $200,000.
The October 2017 Eagle Back-End Note may not be prepaid by the Company.
| F-52 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
Upon an event of default, principal and accrued interest will become immediately due and payable under the notes. Additionally, upon an event of default, both notes will accrue interest at a default interest rate of 24% per annum or the highest rate of interest permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
December 29, 2017 Securities Purchase Agreement
The Company entered into an executory contract on December 29, 2017, whereby the Company entered into a securities purchase agreement with Eagle Equities, pursuant to which Eagle Equities purchased a convertible promissory note (the “December 2017 Eagle Note”) from the Company in the aggregate principal amount of $532,435, with principal and the interest thereon convertible into shares of the Company’s common stock at the option of Eagle Equities at any time. The transactions closed on January 2, 2018.
The December 2017 Eagle Note contains an original issue discount of $25,354 such that the purchase price was $507,081. The maturity date of the December 2017 Eagle Note is December 29, 2018. The December 2017 Eagle Note bears interest at a rate of 8% per annum, which interest shall be paid by the Company to Eagle Equities in shares of the Company’s common stock upon receipt of a notice of conversion by the Company from Eagle Equities at any time. The Company has recorded $21,006 of accrued interest as of June 30, 2018 for the December 2017 Eagle Note and total principal outstanding as of June 30, 2018 under the December 2017 Eagle Note was $532,435.
Eagle Equities has the option to convert all or any amount of the principal face amount of the December 2017 Eagle Note, at any time, for shares of the Company’s common stock at a price equal to 60% of the lowest closing bid price of the Company’s common stock as reported on the OTC Markets quotation system for the ten prior trading days, including the day upon which the Company receives a notice of conversion from Eagle Equities. The note is treated as stock settled debt under ASC 480 and accordingly the Company recorded a $354,956 put premium.
The Company used all of the proceeds from the December 2017 Eagle Note to pay off the remainder of its outstanding debt owed to Delafield, an affiliate of Magna Invests, as previously disclosed.
June 14, 2018 Securities Purchase Agreement
Effective June 14, 2018, the Company entered into a securities purchase agreement with Eagle Equities, pursuant to which Eagle Equities purchased a convertible promissory note (the “June 2018 Eagle Note”) from the Company in the aggregate principal amount of $105,000.00, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Eagle Equities any time after the six-month anniversary of the June 2018 Eagle Note. The transactions contemplated by the Purchase Agreement closed on June 19, 2018. Pursuant to the terms of the Purchase Agreement, Eagle Equities deducted $5,000.00 from the principal payment due under the June 2018 Eagle Note, at the time of closing, to be applied to its legal expenses.
The maturity date of the June 2018 Eagle Note is June 14, 2019. The June 2018 Eagle Note bears interest at a rate of 8% per annum, which interest shall be paid by the Company to Eagle Equities in shares of the Company’s common stock upon receipt of a notice of conversion by the Company from Eagle Equities at any time after the six-month anniversary of the June 2018 Eagle Note.
Additionally, Eagle Equities has the option to convert all or any amount of the principal face amount of the June 2018 Eagle Note, at any time, for shares of the Company’s common stock at a price equal to 60% of the lowest closing bid price of the Company’s common stock as reported on the OTC quotation system for the ten prior trading days, including the day upon which the Company receives a notice of conversion from Eagle Equities. However, in the event that the Company’s common stock is restricted by the Depository Trust Company (“DTC”) for any reason, the Conversion Price shall be lowered to 50% of the lowest closing bid price for the duration of such restriction. If the Company fails to maintain a reserve of shares of its common stock at least four times the number of shares issuable upon conversion of the Note for at least 60 days after the issuance of the Note, the conversion discount shall be increased by 10%. Notwithstanding the foregoing, Eagle Equities shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Eagle Equities and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock. The June 2018 Eagle Note is treated as stock settled debt under ASC 480 and accordingly, the Company recorded a $70,000 put premium. The Company has recorded $368 of accrued interest and the total principal outstanding under the June 2018 Eagle Note was $150,000 as of June 30, 2018.
| F-53 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
The June 2018 Eagle Note may be prepaid by the Company with certain penalties until December 11, 2018.
Upon an event of default, principal and accrued interest will become immediately payable under the note. Interest on the outstanding principal shall accrue at a default interest rate of 24% per annum or at the highest rate permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
The total principal amount outstanding under the above Eagle Equities finance agreements, specifically the December 12, 2016, December 21, 2016, January 27, 2017, the March 1, 2017, the August 9, 2017, October 25, 2017, December 29, 2017, and the June 14, 2018 agreements was $1,867,935 as of June 30, 2018 and accrued interest totaled $107,726.
GS Capital Financing Agreements
May 26, 2017 Securities Purchase Agreement
On May 26, 2017, the Company entered into a Securities Purchase Agreement with GS Capital Partners, LLC (“GS Capital”), dated as of May 17, 2017, pursuant to which GS Capital purchased an 8% convertible redeemable junior subordinated promissory note of the Company in the principal amount of $160,000. The note matured on May 26, 2018, upon which any outstanding principal and interest is due and payable. The note may be prepaid with certain penalties within 180 days of issuance. The amounts funded plus accrued interest are convertible at any time after 180 days into common stock at a conversion price equal to 62% of the lowest closing bid price of the Company’s common stock for the ten trading days prior to the conversion, including the date upon which the conversion notice was received by the Company, subject to adjustment in certain events. The note is treated as stock settled debt under ASC 480 and accordingly the Company recorded a $98,065 put premium. As of June 30, 2018, the outstanding principal under the note along with $7,499 of accrued interest was fully converted (see Note 8 – Stockholders’ Deficit) and the repayment resulted in a full reduction of the put premium.
July 24, 2017 Securities Purchase Agreement
On July 24, 2017, the Company entered into a Securities Purchase Agreement with GS Capital, pursuant to which GS Capital purchased two 8% convertible redeemable junior subordinated promissory notes, each in the principal amount of $160,000. The first note (the “July 2017 GS Note”) was funded with cash and the second note (the “July 2017 GS Back-End Note”) was initially paid for by an offsetting promissory note issued by GS Capital to the Company (the “July 2017 GS Note Receivable”). The terms of the July 2017 GS Back-End Note required cash funding prior to any conversion thereunder. The July 2017 GS Note Receivable was due March 24, 2018, unless certain conditions were not met, in which case both the July 2017 GS Back-End Note and the July 2017 GS Note Receivable may both be cancelled. Both the July 2017 GS Note and the July 2017 GS Back-End Note matured on July 24, 2018 upon which any outstanding principal and interest is due and payable. The amounts cash funded plus accrued interest under both the July 2017 GS Note and the July 2017 GS Back-End Note are convertible into common stock of the Company at a conversion price equal to 62% of the lowest closing bid price of the common stock for the ten trading days prior to the conversion, subject to adjustment in certain events. On January 25, 2018, the Company received payment of the July 2017 GS Note Receivable in the amount of $160,000 that offset the July 2017 GS Back-End Note. Proceeds from the July 2017 GS Note Receivable of $8,000 were paid directly to legal fees resulting in net cash proceeds of $152,000 received by the Company. As a result, the July 2017 GS Back-End Note is now convertible. The July 2017 GS Note and the July 2017 GS Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company recorded a $98,065 put premium as each of the notes was funded. As of June 30, 2018, the outstanding principal under the July 2017 Eagle Note and $8,169 of accrued interest was fully converted into shares of the Company’s common stock (see Note 8 – Stockholders’ Deficit) and the repayment resulted in the full reduction of the put premium. The Company has recorded $1,169 of accrued interest as of June 30, 2018 for the July 2017 GS Back-End Note. Total principal outstanding under the July 2017 GS Back-End Note as of June 30, 2018 was $35,000. As of the date of filing of this Annual Report the remaining balance of the July 2017 GS Back-End Note along with accrued interest of $1,281 was fully converted.
| F-54 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
September 21, 2017 Securities Purchase Agreement
On September 21, 2017, the Company entered into Securities Purchase Agreements, with GS Capital, dated as of September 12, 2017, pursuant to which GS Capital purchased two 8% convertible redeemable junior subordinated promissory notes, each in the principal amount of $160,000. The first note (the “September 2017 GS Note”) was funded with cash and the second note (the “September 2017 GS Back-End Note”) was initially paid for by an offsetting promissory note issued by GS Capital to the Company (the “September 2017 GS Note Receivable”). The terms of the September 2017 GS Back-End Note require cash funding prior to any conversion thereunder. The September 2017 GS Note Receivable was due March 24, 2018, unless certain conditions are not met, in which case both the September 2017 GS Back-End Note and the September 2017 GS Note Receivable may both be cancelled. Both the September 2017 GS Note and the September 2017 GS Back-End Note matured on September 12, 2018, upon which any outstanding principal and interest is due and payable. The amounts cash funded plus accrued interest under both the September 2017 GS Note and the September 2017 GS Back-End Note are convertible into common stock of the Company at a conversion price equal to 62% of the lowest closing bid price of the common stock for the ten trading days prior to the conversion, subject to adjustment in certain events. On February 27, 2018, the Company received payment of the September 2017 GS Note Receivable in the amount of $160,000 that offset the September 2017 GS Back-End Note. Proceeds from the September 2017 GS Note Receivable of $8,000 were paid directly to legal fees resulting in net cash proceeds of $152,000 received by the Company. As a result, the September 2017 GS Back-End Note is now convertible. The September 2017 GS Note and the September 2017 GS Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company recorded a $98,065 put premium as each of the notes was funded. The Company has recorded $8,833 of accrued interest as of June 30, 2018 for the September 2017 GS Note. Total principal outstanding under the September 2017 GS Note as of June 30, 2018 was $130,000, as $30,000 was converted during the year ended June 30, 2018 (see Note 8 – Stockholders’ Deficit). The Company has recorded $4,313 of accrued interest as of June 30, 2018 for the September 2017 GS Back-End Note. Total principal outstanding under the September 2017 GS Back-End Note as of June 30, 2018 was $160,000.
The September 2017 GS Back-End Note may not be prepaid by the Company.
Upon an event of default, principal and accrued interest will become immediately due and payable under the notes. Additionally, upon an event of default, both notes will accrue interest at a default interest rate of 24% per annum or the highest rate of interest permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
March 23, 2018 Securities Purchase Agreement
On March 23, 2018, the Company entered into a securities purchase agreement with GS Capital, pursuant to which GS Capital purchased two 8% convertible redeemable junior subordinated promissory notes of the Company, each in the principal amount of $106,000. The first note (the “March 2018 GS Note”) was funded with cash and the second note (the “March 2018 GS Back-End Note”) was initially paid for by an offsetting promissory note issued by GS Capital to the Company (the “March 2018 GS Note Receivable”). The terms of the March 2018 GS Back-End Note require cash funding prior to any conversion thereunder. The March 2018 GS Note Receivable is due November 23, 2018, unless certain conditions are not met, in which case both the March 2018 GS Back-End Note and the March 2018 GS Note Receivable may both be cancelled. Both the March 2018 GS Note and the March 2018 GS Back-End Note mature on March 23, 2019, upon which any outstanding principal and interest is due and payable. The amounts cash funded plus accrued interest under both the March 2018 GS Note and the March 2018 GS Back-End Note are convertible into shares of common stock of the Company at a conversion price equal to 62% of the lowest closing bid price of the common stock for the ten trading days prior to the conversion, subject to adjustment in certain events. On May 31, 2018, the Company received payment of the March 2018 GS Note Receivable in the amount of $106,000 that offset the March 2018 GS Back-End Note. Proceeds from the March 2018 GS Note Receivable of $5,300 were paid directly to legal fees resulting in net cash proceeds of $100,700 received by the Company. As a result, the March 2018 GS Back-End Note is now convertible. The March 2018 GS Note and the March 2018 GS Back-End Note are treated as stock settled debt under ASC 480 and accordingly the Company recorded a $64,968 put premium as each of the notes was funded. The Company has recorded $2,300 of accrued interest as of June 30, 2018 for the March 2018 GS Note. Total principal outstanding under the March 2018 GS Note as of June 30, 2018 was $106,000. The Company has recorded $697 of accrued interest as of June 30, 2017 for the March 2018 GS Back-End Note. Total principal outstanding under the March 2018 GS Back-End Note as of June 30, 2018 was $106,000.
| F-55 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
The March 2018 GS Note may be prepaid by the Company with certain penalties within 180 days of issuance. The March 2018 GS Back-End Note may not be prepaid by the Company.
Upon an event of default, principal and accrued interest will become immediately due and payable under the notes. Additionally, upon an event of default, both notes will accrue interest at a default interest rate of 24% per annum or the highest rate of interest permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
April 13, 2018 Securities Purchase Agreement
On April 13, 2018, the Company entered into a securities purchase agreement with GS Capital, pursuant to which GS Capital purchased two 8% unsecured convertible promissory notes (the “April 2018 GS Notes”) from the Company each in the principal amount of $150,000. The first note (the “April 2018 GS Note”) was funded with cash and the second note (the “April 2018 GS Back-End Note”) was initially paid for by an offsetting promissory note issued by GS Capital to the Company (the “April 2018 GS Note Receivable”). The terms of the April 2018 Back-End Note require cash funding prior to any conversion thereunder.
Both the April 2018 GS Note and the April 2018 GS Back-End Note mature on April 13, 2019, upon which any outstanding principal and interest thereon is due and payable. The amounts cash funded plus accrued interest under both the April 2018 GS Note and the April 2018 GS Back-End Note are convertibles into shares of the Company’s common stock, at any time after October 13, 2018, at a conversion price for each share of common stock equal to 61% of the lowest closing bid price of the Company’s common stock for the ten prior trading days including the day upon which a notice of conversion is received by the Company from GS Capital, subject to adjustment in certain events. The April 2018 GS Note is treated as stock settled debt under ASC 480 and accordingly the Company recorded a $95,902 put premium. The Company has recorded $2,564 of accrued interest as of June 30, 2018 for the April 2018 GS Note. Total principal outstanding under the April 2018 GS Note as of June 30, 2018 was $150,000.
The April 2018 GS Note may be prepaid until 180 days from the issuance date with certain penalties. The April 2018 GS Back-End Note may not be prepaid. However, in the event that the April 2018 GS Back-End Note has not been cash paid and the April 2018 GS Note is redeemed within the first six months of issuance, the April 2018 GS Back-End Note will be deemed cancelled and of no further effect. The April 2018 GS Back-End Note is not convertible until it is funded in cash on or before December 13, 2018, subject to certain restrictions. The Company has reserved 7,684,000 shares of its common stock for conversions under the April 2018 GS Note.
The April 2018 GS Notes contain certain events of default, upon which principal and accrued interest will become immediately due and payable. In addition, upon an event of default, interest on the outstanding principal shall accrue at a default interest rate of 24% per annum, or if such rate is usurious or not permitted by current law, then at the highest rate of interest permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
The total principal amount outstanding under the above GS Capital finance agreements, specifically the May 26, 2017, July 24, 2017, September 21, 2017, the March 23, 2018 and the April 13, 2018 agreements, was $687,000 as of June 30, 2018 and accrued interest thereunder totaled $19,877.
Regal Consulting Agreements
November 2016 Consulting Agreement
On November 18, 2016 (the “Effective Date”), the Company entered into a consulting agreement with Regal Consulting, LLC (the “Consultant”) for strategic and business advisory services. As compensation for services rendered, the Company issued Consultant two fully earned $250,000 convertible junior subordinated promissory notes. Both notes have a two-year maturity date and interest of 10% per annum. Both notes are junior and subordinate in all respects to the existing debt of the Company. These notes may not be prepaid without the written consent of the Consultant.
| F-56 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
The Company issued the first $250,000 convertible note on November 18, 2016. This note is convertible at a conversion price of the lesser of $2.50 or 65% of the average of the three lowest 10 trading days prior to the conversion. An aggregate total of $255,757 of this note was bifurcated with the embedded conversion option recorded as a derivative liability at fair value. During the year ended June 30, 2017, $27,500 of principal and accrued interest of $1,664 was converted into shares of the Company’s common stock. As of June 30, 2018, the outstanding principal balance of the note along with $19,639 of accrued interest was converted into shares of the Company’s common stock (See Note 8 – Stockholders’ Deficit).
The Company issued the second $250,000 convertible note on February 16, 2017. This note is convertible at a conversion price of the lesser of $2.50 or 65% of the average of the three lowest 10 trading days prior to the conversion. An aggregate total of $409,416 of this note was bifurcated with the embedded conversion option recorded as a derivative liability at fair value. As of June 30, 2018, the outstanding principal balance of the note along with $31,021 of accrued interest was converted into shares of the Company’s common stock (see Note 8 – Stockholders’ Deficit).
August 10, 2017 Consulting Agreement
On August 10, 2017, the Company entered into an agreement, retroactive to May 16, 2017, with the Consultant, pursuant to which the Consultant agreed to provide certain consulting and business advisory services in exchange for a $310,000 junior subordinated convertible note. The note accrues interest at a rate of 10% per annum and is convertible into common stock at the lesser of $1.50 or 65% of the three lowest trades in the ten trading days prior to the conversion. The note was fully earned upon signing the agreement and matures on August 10, 2019. This note may not be prepaid without the written consent of the Consultant. The Company accrued $155,000 related to this expense at June 30, 2017 and recorded the remaining $155,000 related to this expense in fiscal 2018. Upon an event of default, principal and accrued interest will become immediately due and payable under the Consulting Note. Additionally, upon an event of default the note would accrue interest at a default interest rate of 18% per annum or the highest rate of interest permitted by law. The agreement had a three-month term and expired on August 16, 2017. An aggregate total of $578,212 of this note was bifurcated with the embedded conversion option recorded as a derivative liability at fair value (See Note 12 - Derivative Financial Instruments and Fair Value Measurements). During the year ended June 30, 2018, the consultant converted $140,000 of principal and $10,764 of interest (see Note 8 – Stockholders’ Deficit) such that the remaining principal outstanding and accrued interest under this note as of June 30, 2018 was $170,000 and $14,335, respectively.
Power Up Lending Group Finance Agreements
January 22, 2018 Securities Purchase Agreement
Effective January 22, 2018, the Company entered into a securities purchase agreement with Power Up Lending Group Ltd. (“Power Up”), pursuant to which Power Up purchased a convertible promissory note (the “January 2018 Power Up Note”) from the Company in the aggregate principal amount of $153,000, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Power Up. The transaction closed on January 25, 2018 and the Company received payment on January 29, 2018 in the amount of $153,000, of which $2,500 was paid directly toward legal fees and $500 to Power Up for due diligence fees resulting in net cash proceeds of $150,000.
The maturity date of the January 2018 Power Up Note is January 22, 2019. The January 2018 Power Up Note bears interest at a rate of 8% per annum, which interest may be paid by the Company to Power Up in shares of the Company’s common stock, but shall not be payable until the January 2018 Power Up Note becomes payable, whether at the maturity date or upon acceleration or by prepayment. An aggregate total of $180,251 of this note was bifurcated with the embedded conversion option recorded as a derivative liability at fair value (See Note 12 – Derivative Financial Instruments and Fair Value Measurements).
| F-57 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
Additionally, Power Up has the option to convert all or any amount of the principal face amount of the January 2018 Power Up Note, starting on July 21, 2018 and ending on the later of the maturity date and the date the Default Amount, which is an amount equal to 150% of an amount equal to the then outstanding principal amount of the January 2018 Power Up Note plus any interest accrued, is paid if an event of default occurs, for shares of the Company’s common stock at the then-applicable conversion price.
The conversion price for the January 2018 Power Up Note shall be $0.065, subject to certain Market Price (as defined below) adjustment. If the Market Price is greater than or equal to $0.10, the conversion price shall be the greater of 65% of the Market Price (“Variable Conversion Price”) and $0.065. In the event Market Price is less than $0.10, the conversion price shall be the Variable Conversion Price. As defined in the January 2018 Power Up Note, the “Market Price” shall be the average of the lowest three closing bid prices during the ten day trading period prior to and including the day the Company receives a notice of conversion from Power Up on the electronic quotation system or applicable principal securities exchange or trading market or, if no closing bid price of such security is available in any of the foregoing manners, the average of the closing bid prices of any market makers for such security that are listed in the “pink sheets” during the ten prior trading days, including the day upon which the Company receives a notice of conversion from Power Up. Notwithstanding the foregoing, Power Up shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Power Up and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock.
The January 2018 Power Up Note may be prepaid by the Company within 180 days of issuance with certain penalties.
The Company has recorded $5,332 of accrued interest as of June 30, 2018 for the January 2018 Power Up Note. Total principal outstanding under the January 2018 Power Up Note as of June 30, 2018 was $153,000.
March 5, 2018 Securities Purchase Agreement
On March 5, 2018, the Company entered into a securities purchase agreement with Power Up, pursuant to which Power Up purchased a convertible promissory note (the “March 2018 Power Up Note”) from the Company in the aggregate principal amount of $53,000, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Power Up. The Company received payment on March 12, 2018 in the amount of $53,000, of which $2,500 was paid directly toward legal fees and $500 to Power Up for due diligence fees resulting in net cash proceeds of $50,000.
The maturity date of the March 2018 Power Up Note is March 5, 2019. The March 2018 Power Up Note shall bear interest at a rate of 8% per annum, which interest may be paid by the Company to Power Up in shares of the Company’s common stock, but shall not be payable until the March 2018 Power Up Note becomes payable, whether at the maturity date or upon acceleration or by prepayment. An aggregate total of $65,231 of this note was bifurcated with the embedded conversion option recorded as a derivative liability at fair value (See Note 12 - Derivative Financial Instruments and Fair Value Measurements).
Additionally, Power Up has the option to convert all or any amount of the principal face amount of the March 2018 Power Up Note, starting on September 1, 2018 and ending on the later of the maturity date and the date the Default Amount, which is an amount equal to 150% of an amount equal to the then outstanding principal amount of the March 2018 Power Up Note plus any interest accrued, is paid if an event of default occurs, for shares of the Company’s common stock at the then-applicable conversion price.
The conversion price for the March 2018 Power Up Note shall be $0.065, subject to certain Market Price (as defined below) adjustment. If the Market Price is greater than or equal to $0.10, the conversion price shall be the greater of 65% of the Market Price (the “Variable Conversion Price”) and $0.065. In the event Market Price is less than $0.10, the conversion price shall be the Variable Conversion Price. As defined in the March 2018 Power Up Note, the “Market Price” shall be the average of the lowest three closing bid prices during the ten day trading period prior to and including the day the Company receives a notice of conversion from Power Up on the electronic quotation system or applicable principal securities exchange or trading market or, if no closing bid price of such security is available in any of the foregoing manners, the average of the closing bid prices of any market makers for such security that are listed in the “pink sheets” during the ten prior trading days, including the day upon which the Company receives a notice of conversion from Power Up. Notwithstanding the foregoing, Power Up shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Power Up and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock.
| F-58 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
The March 2018 Power Up Note may be prepaid by the Company within 180 days of issuance with certain penalties.
The Company has recorded $1,359 of accrued interest as of June 30, 2018 for the March 2018 Power Up Note. Total principal outstanding under the March 2018 Power Up Note as of June 30, 2018 was $53,000.
May 15, 2018 Securities Purchase Agreement
On May 15, 2018, the Company entered into a securities purchase agreement with Power Up, pursuant to which Power Up purchased a convertible promissory note (the “May 2018 Power Up Note”) from the Company in the aggregate principal amount of $53,000, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Power Up. The Company received payment on May 18, 2018 in the amount of $53,000, of which $2,500 was paid directly toward legal fees and $500 to Power Up for due diligence fees resulting in net cash proceeds of $50,000.
The maturity date of the May 2018 Power Up Note is May 5, 2019. The May 2018 Power Up Note shall bear interest at a rate of 8% per annum, which interest may be paid by the Company to Power Up in shares of common stock, but shall not be payable until the May 2018 Power Up Note becomes payable, whether at the maturity date or upon acceleration or by prepayment. An aggregate total of $33,744 of this note was bifurcated with the embedded conversion option recorded as a derivative liability at fair value (See Note 12 Derivative Financial Instruments and Fair Value Measurements).
Additionally, Power Up has the option to convert all or any amount of the principal face amount of the May 2018 Power Up Note, starting on November 11, 2018 and ending on the later of the maturity date and the date the Default Amount, which is an amount equal to 150% of an amount equal to the then outstanding principal amount of the May 2018 Power Up Note plus any interest accrued, is paid if an event of default occurs, for shares of the Company’s common stock at the then-applicable conversion price.
The conversion price for the May 2018 Power Up Note shall be $0.065, subject to certain Market Price (as defined below) adjustment. If the Market Price is greater than or equal to $0.10, the conversion price shall be the greater of 65% of the Market Price (“Variable Conversion Price”) and $0.065. In the event Market Price is less than $0.10, the conversion price shall be the Variable Conversion Price. As defined in the May 2018 Power Up Note, the “Market Price” shall be the average of the lowest three closing bid prices during the ten day trading period prior to and including the day the Company receives a notice of conversion from Power Up on the electronic quotation system or applicable principal securities exchange or trading market or, if no closing bid price of such security is available in any of the foregoing manners, the average of the closing bid prices of any market makers for such security that are listed in the “pink sheets” during the ten prior trading days, including the day upon which the Company receives a notice of conversion from Power Up. Notwithstanding the foregoing, Power Up shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Power Up and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock.
The May 2018 Power Up Note may be prepaid by the Company within 180 days of issuance with certain penalties.
The Company has recorded $174 of accrued interest as of June 30, 2018 for the May 2018 Power Up Note. Total principal outstanding under the May 2018 Power Up Note as of June 30, 2018 was $53,000.
JSJ Investments, Inc. Finance Agreement
Effective June 26, 2018, the Company issued a convertible promissory note (the “June 2018 JSJ Note”) to JSJ Investments, Inc. (“JSJ”) in the aggregate principal amount of $113,000, with principal and the interest thereon convertible into shares of the Company’s common stock at the option of JSJ any time after 180 days of issuance. At the time of closing on June 27, 2018, JSJ deducted $3,000 from the principal payment due under the June 2018 JSJ Note to be applied to its legal expenses, such that the Company received aggregate net proceeds of $110,000 at closing.
| F-59 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
The maturity date of the June 2018 JSJ Note is June 26, 2019, unless extended for up to one year at JSJ’s discretion (the “Maturity Date”). The June 2018 JSJ Note bears interest at a rate of 8% per annum, and after the maturity date shall compound quarterly.
Additionally, JSJ has the option to convert all or any amount of the principal face amount of the June 2018 JSJ Note, at any time beginning December 23, 2018, for shares of the Company’s common stock at a price equal to 65% of the lowest closing bid price (the “Closing Bid Price”) of the Company’s common stock as reported on the OTC Markets quotation system for the ten prior trading days, including the day upon which the Company receives a notice of conversion from JSJ (the “Conversion Price’). However, in the event that the Company’s common stock is restricted by the DTC for any reason, the Conversion Price shall be lowered to 50% of the lowest Closing Bid Price for the duration of such restriction. Notwithstanding the foregoing, JSJ shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by JSJ and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock. The June 2018 JSJ Note is treated as stock settled debt under ASC 480 and accordingly the Company recorded a $60,846 put premium.
The June 2018 JSJ Note may be prepaid until December 23, 2018. If the June 2018 JSJ Note is prepaid within 90 days of the issuance date, then the prepayment premium shall be 135% of the face amount plus any accrued interest; if the JSJ Note is prepaid after 90 days from the issuance date, but prior to 121 days from the issuance date, then the prepayment premium shall be 140% of the face amount plus any accrued interest; and if the June 2018 JSJ Note is prepaid after 120 days from the issuance date, but prior to 180 days from the issuance date, then the prepayment premium shall be 145% of the face amount plus any accrued interest.
The Company shall at all times reserve a minimum of four times the number of shares required if all outstanding principal, and interest under the June 2018 JSJ Note would be fully converted, and JSJ may reasonably request increases from time to time to reserve share amounts.
The June 2018 JSJ Note contains certain events of default, including failure to timely issue shares upon receipt of a notice of conversion, failure to give at least 20 days notice of a reverse split, and failure to file all reports required to be filed by it with the SEC or the OTC Markets to remain a “Current Information” designated company, as well as certain customary events of default, including, among others, a breach of the covenants, insolvency, bankruptcy and failure by the Company to pay the principal and interest due under the June 2018 JSJ Note.
Upon an event of default, interest on the outstanding principal shall accrue at a default interest rate of 18% per annum or at the highest rate permitted by law. Amounts due under the June 2018 JSJ Note in the event of a default shall be based on the value of the underlying conversion shares and calculated off of the highest price of the Company’s common stock at any time between June 26, 2018 and the date of the event of default. In addition, for the first three occurrences of an event of default, the conversion discount shall be increased by 5% for each occurrence of a default.
The Company has recorded $124 of accrued interest as of June 30, 2018 for the June 2018 JSJ Note. Total principal outstanding under the June 2018 JSJ Note as of June 30, 2018 was $113,000.
The Company recorded $543,744 and $650,000 of debt discounts related to the above note issuances during the years ended June 30, 2018 and 2017, respectively. The debt discounts are being amortized over the term of the debt. Amortization of all debt discounts for the years ended June 30, 2018 and 2017 was $853,459 and $1,958,515, respectively.
See Note 13- Subsequent Events for information about financings since the conclusion of the fiscal year.
NOTE 7 – INCOME TAXES
The Company follows ASC 740-10-10, under which an entity recognizes deferred tax assets and liabilities for future tax consequences or for events that were previously recognized in the Company’s financial statements or tax returns. The measurement of deferred tax assets and liabilities is based on enacted tax law provisions. The effects of future changes in tax laws or rates are not anticipated. Through June 30, 2010, the Company operated exclusively in Australia. The Company was wholly subject to Australian income tax laws and regulations, which are administered by the Australian Taxation Office for the years ended June 30, 2010 and all prior years.
| F-60 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
On November 23, 2010, the Company was incorporated in the state of Delaware. In January 2011, the Company acquired all of the outstanding shares of Propanc PTY LTD on a one-for-one basis with Propanc PTY LTD becoming a wholly-owned subsidiary of the Company. As a result of these transactions, the Company is subject to the income tax laws of both the United States and Australia for the years ended June 30, 2013 through June 30, 2018.
For the years ended June 30, 2018 and 2017, the Company’s losses before income taxes resulted from both its Australian and US activities and its taxable losses are subject to both Australian and U.S. tax law. At June 30, 2018, the Company has net operating loss (“NOL”) carryforwards for Australian tax purposes only that is approximately $18,896,000. At June 30, 2018, the Company has NOL carryforwards for US tax purposes only that is approximately $2,767,000. Consequently, the Company may have NOL carryforwards available for income tax purposes that will continue to be available until they are recovered through earning taxable income. Deferred tax assets would arise from the recognition of anticipated utilization of these net operating losses to offset future taxable income. The NOL for Australian tax purposes is subject to a reduction of $4,693,000 for research and development credits granted by the Australian Taxation Office through June 30, 2018.
The components for the provision for income taxes are as follows:
| Year Ended | ||||||||
| June 30, 2018 | June 30, 2017 | |||||||
| Current Taxes | $ | (278,320 | ) | $ | (305,673 | ) | ||
| Deferred Taxes | - | - | ||||||
| Income Taxes Expense (Benefit) | $ | (278,320 | ) | $ | (305,673 | ) | ||
The items accounting for the difference between income taxes at the Australia statutory rate and the provision for income taxes are as follows:
| Year Ended | ||||||||||||||||
| June 30, 2018 | June 30, 2017 | |||||||||||||||
| Amount | Impact on Rate | Amount | Impact on Rate | |||||||||||||
| Income Tax Expense (Benefit) at Australia Statutory Rate | $ | (2,325,482 | ) | (32.22 | )% | $ | (1,830,192 | ) | (22.39 | )% | ||||||
| Expenses Paid by Parent on Behalf of Foreign Subsidiary | 159,944 | 2.22 | % | 922,125 | 11.28 | % | ||||||||||
| R&D Refundable Tax Credit | (179,307 | ) | (2.48 | )% | (305,673 | ) | (3.74 | )% | ||||||||
| Reduction of NOL Carryforward Due to R&D Tax Credit | 179,307 | 2.48 | % | 305,673 | 3.74 | % | ||||||||||
| Change in Federal Tax Rates | (99,013 | ) | (1.37 | )% | - | 0.00 | % | |||||||||
| Change in Deferred Tax Valuation Allowance | 1,005,570 | 13.93 | % | 881,596 | 10.79 | % | ||||||||||
| Foreign Exchange Rate Changes | 980,661 | 13.59 | % | (279,202 | ) | (3.42 | )% | |||||||||
| Total Income Tax Expense (Benefit) | $ | (278,320 | ) | (3.86 | )% | $ | (305,673 | ) | (3.74 | )% | ||||||
| F-61 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and amounts used for income tax purposes. Significant components of the Company’s net deferred income taxes are as follows:
| June 30, 2018 | June 30, 2017 | |||||||
| Current Deferred Tax Assets | ||||||||
| Warrant Derivative Liability | $ | 7,403 | $ | 8,460 | ||||
| Provision for Annual Leave | 44,969 | 36,190 | ||||||
| Superannuation | 4,041 | 99 | ||||||
| Total Current Deferred Tax Assets | $ | 56,413 | $ | 44,749 | ||||
| Current Deferred Tax Liabilities | ||||||||
| Prepaid Investor Services | $ | 18,378 | $ | 16,966 | ||||
| Total Current Deferred Tax Liabilities | $ | 18,378 | $ | 16,966 | ||||
| Non-Current Deferred Tax Assets | ||||||||
| Prepaid Investor Services | $ | 335,175 | $ | 426,664 | ||||
| Capital Raising Costs | 23,559 | 23,325 | ||||||
| Legal Costs | 23,885 | 23,648 | ||||||
| Intellectual Property | 11,760 | 11,643 | ||||||
| Patent Costs | 171,195 | 128,950 | ||||||
| Formation Expense | 7,208 | 6,881 | ||||||
| Net Operating Loss Carryover | 5,668,743 | 4,215,141 | ||||||
| Foreign Exchange Loss (OCI) | (39,379 | ) | (39,379 | ) | ||||
| Total Non-Current Deferred Tax Assets | 6,202,146 | 4,796,873 | ||||||
| Deferred Tax Valuation Allowance | (6,276,937 | ) | (4,858,588 | ) | ||||
| Total Non-Current Deferred Tax Assets | (74,791 | ) | (61,715 | ) | ||||
| Total Deferred Tax Assets (Net) | $ | - | $ | - | ||||
Management has determined that the realization of the net deferred tax asset is not assured and has created a valuation allowance for the entire amount of such benefits.
The Company follows ASC 740-10, which provides guidance for the recognition and measurement of certain tax positions in an enterprise’s financial statements. Recognition involves a determination whether it is more likely than not that a tax position will be sustained upon examination with the presumption that the tax position will be examined by the appropriate taxing authority having full knowledge of all relevant information.
The Company’s policy is to record interest and penalties associated with unrecognized tax benefits as additional income taxes in the statement of operations. As of June 30, 2018 the Company had no unrecognized tax benefits. There were no changes in the Company’s unrecognized tax benefits during the years ended June 30, 2018 and 2017. The Company did not recognize any interest or penalties during fiscal 2018 or 2017 related to unrecognized tax benefits.
The income tax returns filed for the tax years from inception will be subject to examination by the relevant taxing authorities.
| F-62 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
NOTE 8 – STOCKHOLDERS’ DEFICIT
Reverse Stock Split
On April 20, 2017, the Company effected a one-for-two hundred and fifty (1:250) reverse stock split whereby the Company (i) decreased the number of its authorized shares of common stock, par value $0.001 per share, to 100,000,000 (ii) decreased the number of authorized shares of preferred stock to 1,500,005, and (iii) decreased, by a ratio of one-for-two hundred and fifty (1:250) the number of issued and outstanding shares of its common stock. Proportional adjustments for the reverse stock split were made to the Company’s outstanding stock options, warrants and equity incentive plans, including all share and per-share data, for all amounts and periods presented in the Company’s consolidated financial statements included elsewhere in this Annual Report.
Preferred Stock:
As of the date of this Annual Report, the total number of shares of preferred stock that the Company is authorized to issue is 1,500,005, $0.01 par value per share. These preferred shares have no rights to dividends, profit sharing or liquidation preferences.
Of the total preferred shares authorized, 500,000 have been designated as Series A Preferred Stock, $0.01 par value per share (“Series A Preferred Stock”), pursuant to the Certificate of Designation filed with the Secretary of State of the State of Delaware on December 9, 2014. 500,000 shares of Series A Preferred Stock are issued and outstanding as of June 30, 2018.
Of the total preferred shares authorized, pursuant to the Certificate of Designation filed with the Secretary of State of the State of Delaware on June 16, 2015, up to five shares have been designated as Series B Preferred Stock, $0.01 par value per share (“Series B Preferred Stock”). Each holder of outstanding shares of Series B Preferred Stock is entitled to voting power equivalent to the number of votes equal to the total number of shares of common stock outstanding as of the record date for the determination of stockholders entitled to vote at each meeting of stockholders of the Company and entitled to vote on all matters submitted or required to be submitted to a vote of the stockholders of the Company. One share of Series B Preferred Stock is issued and outstanding as of June 30, 2018.
No shares of Series A Preferred Stock or Series B Preferred Stock were issued in fiscal years 2018 or 2017.
Common Stock:
Shares Issued for Services
On July 14, 2016, the Company agreed with a certain consultant to an addendum to two consulting agreements entered into on May 7, 2015 and April 22, 2016, respectively. The Company then owed the consultant $60,000 related to the May 7, 2015 agreement for monthly consulting fees and $100,000 related to the April 22, 2016 agreement, which was comprised of a $10,000 retainer and $90,000 for three reports issued by the consultant. The Company agreed to issue 24,000 shares of its common stock in consideration of the $60,000 in outstanding fees related to the May 7, 2015 agreement and an additional 24,000 shares in forgiveness of future monthly consulting fees, valued at $95,400. In addition, the Company agreed to issue 40,000 shares of its common stock in consideration for the $100,000 in outstanding fees related to the April 22, 2016 agreement. The shares were issued on November 4, 2016 and an additional loss on settlement of debt was recorded of $94,400 based on the fair market value of $349,800 for 88,000 shares on July 14, 2016 (a share price of $3.98).
On June 29, 2017, the above May 7, 2015 agreement was further amended and the Company agreed to issue the consultant 100,000 shares of the Company’s common stock as consideration to eliminate the monthly retainer fee of $7,500 on a going forward basis and to waive the consultant’s right of first refusal (the “Right of First Refusal”) to act as lead book running manager of any public or private offering of securities or any other financing during the term of the engagement. The shares were valued based on the closing price on the date of the agreement at $0.95 or $95,000, which was recognized as consulting expense for the year ended June 30, 2017. The 100,000 shares were issued on July 25, 2017.
| F-63 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
On October 1, 2015, the Company entered into an agreement with a certain consultant to provide services to the Company over a one-year period. The Company agreed to issue the consultant 6,000 shares of its common stock and an additional 6,000 shares of its common stock on April 1, 2016 unless the Company terminated the agreement before then. The Company valued the 6,000 initial shares based on the market price on the agreement date of $7.75 per share and recognized $46,500 of consulting expense over the one-year term of the agreement. The Company recorded $34,907 of consulting expense for the year ended June 30, 2016 related to this agreement. On October 1, 2015, the Company issued 4,400 and 1,600 shares of its common stock to the consultant related to this agreement. In February 2016, the Company terminated this agreement and the remaining $11,593 was recorded as consulting expense in the year ended June 30, 2017.
On November 1, 2015, the Company entered into an agreement with a certain consultant to provide services to the Company over a nine-month period. The Company agreed to issue the consultant 8,480 shares of the Company’s common stock. The Company recorded $28,305 of consulting expense for the year ended June 30, 2016 related to this agreement. On August 8, 2016, the Company issued the 8,480 shares of common stock valued at $3.75 per share to the consultant.
On December 30, 2015, the Company entered into an agreement with a certain consultant to provide services to the Company over a nine-month period. The Company agreed to issue the consultant 4,000 shares of the Company’s common stock. The Company valued the 4,000 shares based on the market price of common stock on the agreement date of $6.50 and recognized $26,000 of consulting expense over the term of the agreement. On January 4, 2016, the Company issued the 4,000 shares of its common stock pursuant to this agreement. The Company recorded $17,271 of consulting expense for the year ended June 30, 2016 and the remaining $8,279 in the year ended June 30, 2017 related to this agreement.
On January 31, 2016, the Company entered into an agreement with a certain consultant to provide services over a five-month period in exchange for 36,000 shares of the Company’s common stock. On August 23, 2016, the Company issued the 36,000 shares of the Company’s common stock valued at $2.60 per share to the consultant. These services were expensed during the year ended June 30, 2016.
On October 27, 2016, the Company entered into an agreement with a third party for professional services to the Company over a six-month period commencing on October 10, 2016 in exchange for a monthly fee of $22,500, of which $10,000 a month was to be paid in cash and $12,500 per month in shares of the Company’s common stock. Additionally, the Company acknowledged an existing outstanding balance due of $20,500 for September 2016 services provided by such party to the Company. The Company recorded $75,000 of consulting expense with respect to such shares of its common stock for the year ended June 30, 2017 related to this agreement. On March 2, 2017, the Company issued 30,000 shares of its common stock to such third party as consideration for the $75,000 of consulting expense (at a per share price of $2.50).
On February 1, 2017, the Company received an invoice for $30,000 from a third party for six months of consulting services performed during the period of August 1, 2016 through January 31, 2017. The invoice was payable 50% in cash and 50% in shares of the Company’s common stock. The Company recorded $30,000 in consulting fees related to this invoice for the year ended June 30, 2017. The Company issued 30,000 shares on July 25, 2017 at a per share price of $0.50, or $15,000. The shares were valued at fair market value on January 31, 2017 at $2.63 per share and an additional loss on settlement of debt was recorded of $63,750.
On May 10, 2017, the Company entered into a seven-month agreement from May 10, 2017 through January 10, 2018, excluding August 2017, with a third party for growth strategy consulting services to be provided to the Company, whereby the Company would issue and deliver to the third party, 7,500 shares of its common stock per month as consideration for the services. Shares were to be valued on the 10th day of the month they were earned and as of June 30, 2017, the Company recorded consulting fees for 15,000 shares related to two months of services or $16,050. The contract was terminated in September 2017. In June of 2018, the shares were determined by the Company’s board of directors to be non-issuable due to non-performance and the Company recorded a loss on debt extinguishment of $16,050 related to the shares issued in the prior year.
| F-64 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
On August 1, 2017, the Company received an invoice for $30,000 from a third party for six months of consulting services provided to the Company during the period of February 1, 2017 through July 31, 2017. The invoice was payable in shares of the Company’s common stock. The Company recorded $25,000 in consulting fees related to this invoice for the year ended June 30, 2017 and the balance was recorded in fiscal year 2018. On February 15, 2018, the Company issued 234,375 shares of its common stock and an additional loss on settlement of debt was recorded of $68,438 based on the fair market value on July 31, 2017, when the shares were fully earned, of $0.42 per share.
On December 29, 2017, the Company entered into a one-year consulting agreement with a certain consultant (the “Consultant”) for certain consulting, advisory and media services to be provided to the Company. As compensation for such services, the Company agreed to pay the consultant (i) an hourly fee of $950 per hour, for up to $71,250 of time-based services; (ii) $9,772 for the preparation of certain marketing materials; (iii) an upfront fee of 500,000 restricted shares of the Company’s common stock, with up to 750,000 additional shares to be issued on the six month anniversary of the date of the consulting agreement at the Company’s sole discretion, and (iv) a marketing bonus equal to 6% of the value of any: (x) business collaboration with the Company which is identified or introduced by the Consultant; or (y) joint venture, licensing, collaboration or similar monetization or strategic transaction (other than any capital-raising transaction) which is identified or introduced by the Consultant. The Company could, in its sole discretion, pay any of the aforementioned fees in cash or shares of the Company’s common stock. If such fees are paid in stock, the number of shares to be paid was to be calculated by dividing the dollar amount of time (or value of the transaction, as the case may be) invoiced in such pay period by, as of the applicable calculation date, the most recent price at which the Company has sold shares of its common stock (or securities convertible into common stock) in a bona fide public or private financing including third party investors. The Company valued the 500,000 shares based on the market price on the agreement date of $0.14 and will recognize $70,000 of consulting expense through the term of the agreement. For the year ended June 30, 2018, the Company recorded $35,287 of expense related to this agreement. On February 15, 2018, the Company issued the 500,000 shares to the Consultant.
On February 1, 2018, the Company received an invoice for $30,000 from a third party for six months of consulting services provided to the Company during the period of August 1, 2017 through January 31, 2018. The invoice was payable in shares of the Company’s common stock. The Company issued 234,375 shares on February 15, 2018 and recorded $30,000 in consulting fees during fiscal 2018. An additional loss on settlement of debt was recorded of $2,813 based on the fair market value on January 31, 2018, when the shares were fully earned, of $0.14 per share.
Settlement of Accounts Payable for Shares of Common Stock
On February 13, 2017, the Company entered into an agreement with a third party whereby the Company agreed to issue and deliver to the third party, in lieu of payment of $50,000 of existing accounts payable, shares of the Company’s common stock. On March 2, 2017, the Company issued 16,667 shares of its common stock at a per share price of $3.00 in consideration for the $50,000 in accounts payable.
Shares Issued for Conversion of Convertible Debt
Fiscal Year 2017:
On August 18, 2016, pursuant to a conversion notice, $32,500 of principal and $2,885 of interest was converted at $2.06 per share into 17,156 shares of common stock.
On August 25, 2016, pursuant to a conversion notice, $54,375 of interest was converted at $2.91 per share into 18,710 shares of common stock.
On September 21, 2016, pursuant to a conversion notice, $25,000 of principal was converted at $2.73 per share into 9,151 shares of common stock.
On September 28, 2016, pursuant to a conversion notice, $20,000 of principal was converted at $2.73 per share into 7,321 shares of common stock.
| F-65 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
On September 30, 2016, pursuant to a conversion notice, $17,500 of principal and $1,350 of interest was converted at $1.95 per share into 9,654 shares of common stock.
On October 4, 2016, pursuant to a conversion notice, $25,000 of principal was converted at $2.54 per share into 9,849 shares of common stock.
On October 6, 2016, pursuant to a conversion notice, $1,000 of principal and $79 of interest was converted at $1.77 per share into 608 shares of common stock.
On October 7, 2016, pursuant to a conversion notice, $25,000 of principal was converted at $2.36 per share into 10,576 shares of common stock.
On October 7, 2016, pursuant to a conversion notice, $1,000 of principal and $79 of interest was converted at $1.68 per share into 643 shares of common stock.
On October 14, 2016, pursuant to a conversion notice, $25,000 of principal was converted at $2.36 per share into 10,576 shares of common stock.
On October 19, 2016, pursuant to a conversion notice, $25,000 of principal was converted at $2.03 per share into 12,288 shares of common stock.
On October 21, 2016, pursuant to a conversion notice, $50,000 of principal was converted at $1.94 per share into 25,806 shares of common stock.
On November 9, 2016, pursuant to a conversion notice, $54,375 of interest was converted at $2.07 per share into 26,227 shares of common stock.
On November 21, 2016, pursuant to a conversion notice, $50,000 of principal was converted at $2.03 per share into 24,576 shares of common stock.
On December 2, 2016, pursuant to a conversion notice, $25,000 of principal was converted at $1.88 per share into 13,301 shares of common stock.
On December 8, 2016, pursuant to a conversion notice, $25,000 of principal was converted at $1.30 per share into 19,257 shares of common stock.
On December 8, 2016, pursuant to a conversion notice, $36,500 of principal and $3,368 of interest was converted at $1.06 per share into 37,656 shares of common stock.
On December 9, 2016, pursuant to a conversion notice, $1,000 of principal and $93 of interest was converted at $1.06 per share into 1,032 shares of common stock.
On December 15, 2016, pursuant to a conversion notice, $35,000 of principal was converted at $1.30 per share into 26,959 shares of common stock.
On December 16, 2016, pursuant to a conversion notice, $20,000 of principal and $1,881 of interest was converted at $1.06 per share into 20,666 shares of common stock.
On December 23, 2016, pursuant to a conversion notice, $20,000 of principal was converted at $1.30 per share into 15,405 shares of common stock.
On January 10, 2017, pursuant to a conversion notice, $16,500 of principal and $1,645 of interest was converted at $1.17 per share into 15,526 shares of common stock.
| F-66 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
On January 11, 2017, pursuant to a conversion notice, $136,400 of principal was converted at $1.57 per share into 86,907 shares of common stock.
On January 19, 2017, pursuant to a conversion notice, $36,500 of principal and $3,712 of interest was converted at $1.17 per share into 34,406 shares of common stock.
On January 20, 2017, pursuant to a conversion notice, $31,500 of principal was converted at $1.57 per share into 20,070 shares of common stock.
On January 25, 2017, pursuant to a conversion notice, $55,000 of principal was converted at $1.72 per share into 31,893 shares of common stock.
On February 21, 2017, pursuant to a conversion notice, $75,000 of principal was converted at $1.90 per share into 39,500 shares of common stock.
On April 24, 2017, pursuant to a conversion notice, $25,000 of principal was converted at $0.78 per share into 32,259 shares of common stock.
On May 2, 2017, pursuant to a conversion notice, $10,000 of principal and $402 of interest was converted at $0.90 per share into 11,558 shares of common stock.
On May 5, 2017, pursuant to a conversion notice, $19,386 of principal was converted at $0.78 per share into 25,015 shares of common stock.
On May 5, 2017, pursuant to a conversion notice, $23,114 of principal was converted at $0.78 per share into 29,825 shares of common stock.
On May 8, 2017, pursuant to a conversion notice, $15,000 of principal and $623 of interest was converted at $0.87 per share into 17,958 shares of common stock.
On May 12, 2017, pursuant to a conversion notice, $10,000 of principal and $424 of interest was converted at $0.57 per share into 18,288 shares of common stock.
On May 16, 2017, pursuant to a conversion notice, $20,000 of principal and $867 of interest was converted at $0.57 into 36,608 shares of common stock.
On May 18, 2017, pursuant to a conversion notice, $42,500 of principal was converted at $0.74 per share into 57,725 shares of common stock.
On May 22, 2017, pursuant to a conversion notice, $20,000 of principal and $893 of interest was converted at $0.57 per share into 36,655 shares of common stock.
On May 24, 2017, pursuant to a conversion notice, $25,000 of principal and $1,128 of interest was converted at $0.57 per share into 45,838 shares of common stock.
On May 30, 2017, pursuant to a conversion notice, $42,500 of principal was converted at $0.77 per share into 55,393 shares of common stock.
On June 6, 2017, pursuant to a conversion notice, $25,000 of principal and $317 of interest was converted at $0.67 per share into 38,013 shares of common stock.
On June 16, 2017, pursuant to a conversion notice, $20,000 of principal and $298 of interest was converted at $0.60 per share into 33,830 shares of common stock.
| F-67 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
On June 21, 2017, pursuant to a conversion notice, $42,500 of principal was converted at $0.76 per share into 55,901 shares of common stock.
On June 23, 2017, pursuant to a conversion notice, $25,000 of principal and $411 of interest was converted at $0.60 per share into 42,352 shares of common stock.
On June 23, 2017, pursuant to a conversion notice, $27,500 in principal and $1,604 in interest was converted at $0.64 per share into 45,192 shares of common stock.
On June 26, 2017, pursuant to a conversion notice, $42,500 of principal was converted at $0.76 per share into 55,901 shares of common stock.
On June 28, 2017, pursuant to a conversion notice, $30,000 of principal and $527 of interest was converted at $0.60 per share into 50,878 shares of common stock.
Fiscal 2018:
On July 5, 2017, pursuant to a conversion notice, $26,000 of principal and $1,121 of interest was converted at $0.54 per share into 49,946 shares of common stock.
On July 13, 2017, pursuant to a conversion notice, $42,500 of principal was converted at $0.63 per share into 67,694 shares of common stock.
On July 17, 2017, pursuant to a conversion notice, $16,000 of principal and $732 of interest was converted at $0.40 per share into 41,623 shares of common stock.
On July 20, 2017, pursuant to a conversion notice, $28,000 of principal and $1,300 of interest was converted at $0.29 per share into 101,738 shares of common stock.
On July 28, 2017, pursuant to a conversion notice, $22,500 in principal and $1,593 in interest was converted at $0.26 per share into 93,365 shares of common stock.
On August 2, 2017, pursuant to a conversion notice, $20,000 of principal was converted at $0.28 per share into 70,897 shares of common stock.
On August 2, 2017, pursuant to a conversion notice, $25,000 of principal and $1,233 of interest was converted at $0.21 per share into 124,921 shares of common stock.
On August 16, 2017, pursuant to a conversion notice, $25,000 of principal and $1,311 of interest was converted at $0.23 per share into 112,441 shares of common stock.
On August 17, 2017, pursuant to a conversion notice, $20,000 of principal was converted at $0.30 per share into 66,171 shares of common stock.
On August 22, 2017, pursuant to a conversion notice, $20,000 of principal and $1,500 of interest was converted at $0.25 per share into 84,812 shares of common stock.
On August 25, 2017, pursuant to a conversion notice, $25,000 of principal and $1,361 of interest was converted at $0.23 per share into 112,654 shares of common stock.
On August 29, 2017, pursuant to a conversion notice, $20,000 of principal was converted at $0.24 per share into 81,926 shares of common stock.
On September 3, 2017, pursuant to a conversion notice, $20,000 of principal and $1,661 of interest was converted at $0.20 per share into 106,390 shares of common stock.
| F-68 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
On September 6, 2017, pursuant to a conversion notice, $12,500 of principal and $714 of interest was converted at $0.19 per share into 71,042 shares of common stock.
On September 8, 2017, pursuant to a conversion notice, $20,000 of principal was converted at $0.24 per share into 83,247 shares of common stock.
On September 14, 2017, pursuant to a conversion notice, $15,000 of principal and $450 of interest was converted at $0.15 per share into 103,000 shares of common stock.
On September 14, 2017, pursuant to a conversion notice, $20,000 of principal and $1,665 of interest was converted at $0.16 per share into 138,878 shares of common stock.
On September 18, 2017, pursuant to a conversion notice, $20,000 of principal was converted at $0.19 per share into 107,527 shares of common stock.
On September 25, 2017, pursuant to a conversion notice, $20,000 of principal and $649 of interest was converted at $0.14 per share into 149,630 shares of common stock.
On September 26, 2017, pursuant to a conversion notice, $30,000 of principal was converted at $0.18 per share into 168,303 shares of common stock.
On September 26, 2017, pursuant to a conversion notice, $20,000 of principal and $1,716 of interest was converted at $0.15 per share into 145,257 shares of common stock.
On October 2, 2017, pursuant to a conversion notice, $25,000 of principal and $850 of interest was converted at $0.14 per share into 187,319 shares of common stock.
On October 4, 2017, pursuant to a conversion notice, $40,000 of principal was converted at $0.18 per share into 224,404 shares of common stock.
On October 5, 2017, pursuant to a conversion notice, $20,000 of principal and $1,716 of interest was converted at $0.15 per share into 145,257 shares of common stock.
On October 9, 2017, pursuant to a conversion notice, $30,000 of principal and $1,067 of interest was converted at $0.14 per share into 215,651 shares of common stock.
On October 10, 2017, pursuant to a conversion notice, $45,000 of principal was converted at $0.19 per share into 241,835 shares of common stock.
On October 11, 2017, pursuant to a conversion notice, $20,000 of principal and $1,812 of interest was converted at $0.16 per share into 139,762 shares of common stock.
On October 16, 2017, pursuant to a conversion notice, $20,000 of principal and $1,834 of interest was converted at $0.16 per share into 134,363 shares of common stock.
On October 18, 2017, pursuant to a conversion notice, $25,000 of principal and $939 of interest was converted at $0.13 per share into 196,507 shares of common stock.
On October 19, 2017, pursuant to a conversion notice, $30,000 of principal was converted at $0.16 per share into 193,549 shares of common stock.
On October 23, 2017, pursuant to a conversion notice, $20,000 of principal and $1,884 of interest was converted at $0.11 per share into 198,045 shares of common stock.
| F-69 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
On October 24, 2017, pursuant to a conversion notice, $21,000 of principal and $817 of interest was converted at $0.11 per share into 202,006 shares of common stock.
On October 27, 2017, pursuant to a conversion notice, $15,000 of principal was converted at $0.09 per share into 159,958 shares of common stock.
On October 30, 2017, pursuant to a conversion notice, $8,750 of principal and $352 of interest was converted at $0.07 per share into 144,475 shares of common stock.
On October 30, 2017, pursuant to a conversion notice, $20,000 of principal and $1,902 of interest was converted at $0.07 per share into 300,851 shares of common stock.
On November 2, 2017, pursuant to a conversion notice, $5,000 of principal and $8,250 of interest was converted at $0.09 per share into 155,426 shares of common stock.
On November 6, 2017, pursuant to a conversion notice, $12,750 of principal and $533 of interest was converted at $0.05 per share into 245,158 shares of common stock.
On November 6, 2017, pursuant to a conversion notice, $17,500 of principal was converted at $0.07 per share into 250,897 shares of common stock.
On November 8, 2017, pursuant to a conversion notice, $20,000 in principal and $2,356 in interest was converted at $0.06 per share into 382,153 shares of common stock.
On November 13, 2017, pursuant to a conversion notice, $11,000 in principal and $623 in interest was converted at $0.05 per share into 215,247 shares of common stock.
On November 15, 2017, pursuant to a conversion notice, $20,000 in principal and $2,443 in interest was converted at $0.06 per share into 383,641 shares of common stock.
On November 17, 2017, pursuant to a conversion notice, $15,000 in principal was converted at $0.07 per share into 215,054 shares of common stock.
On November 26, 2017, pursuant to a conversion notice, $20,000 in principal and $2,568 in interest was converted at $0.06 per share into 385,777 shares of common stock.
On November 27, 2017, pursuant to a conversion notice, $20,000 in principal and $1,196 in interest was converted at $0.05 per share into 392,510 shares of common stock.
On December 1, 2017, pursuant to a conversion notice, $20,000 in principal and $802 in interest was converted at $0.06 per share into 372,799 shares of common stock.
On December 6, 2017, pursuant to a conversion notice, $21,000 in principal and $1,297 in interest was converted at $0.05 per share into 412,914 shares of common stock.
On December 8, 2017, pursuant to a conversion notice, $9,900 in principal and $792 in interest was converted at $0.05 per share into 198,000 shares of common stock.
On December 8, 2017, pursuant to a conversion notice, $42,666 in principal was converted at $0.07 per share into 611,699 shares of common stock.
On December 11, 2017, pursuant to a conversion notice, $9,900 in principal and $799 in interest was converted at $0.05 per share into 198,122 shares of common stock.
| F-70 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
On December 11, 2017, pursuant to a conversion notice, $27,000 in principal and $1,142 in interest was converted at $0.06 per share into 504,339 shares of common stock.
On December 11, 2017, pursuant to a conversion notice, $42,666 in principal was converted at $0.07 per share into 611,699 shares of common stock.
On December 15, 2017, pursuant to a conversion notice, $56,758 in principal was converted at $0.08 per share into 732,362 shares of common stock.
On December 18, 2017, pursuant to a conversion notice, $30,000 in principal and $2,467 in interest was converted at $0.07 per share into 478,859 shares of common stock.
On December 19, 2017, pursuant to a conversion notice, $23,000 in principal and $1,013 in interest was converted at $0.07 per share into 368,867 shares of common stock.
On December 21, 2017, pursuant to a conversion notice, $63,000 in principal was converted at $0.08 per share into 789,227 shares of common stock.
On December 22, 2017, pursuant to a conversion notice, $25,000 in principal and $2,078 in interest was converted at $0.06 per share into 429,806 shares of common stock.
On January 2, 2018, pursuant to a conversion notice, $25,000 in principal and $1,178 in interest was converted at $0.07 per share into 402,121 shares of common stock.
On January 3, 2018, pursuant to a conversion notice, $25,200 in principal and $2,162 in interest was converted at $0.06 per share into 434,311 shares of common stock.
On January 4, 2018, pursuant to a conversion notice, $25,000 in principal and $1,372 in interest was converted at $0.07 per share into 398,854 shares of common stock.
On January 9, 2018, pursuant to a conversion notice, $40,000 in principal and $4,581 in interest was converted at $0.07 per share into 630,384 shares of common stock.
On January 12, 2018, pursuant to a conversion notice, $25,000 in principal and $1,233 in interest was converted at $0.08 per share into 345,396 shares of common stock.
On January 12, 2018, pursuant to a conversion notice, $7,500 in principal and $875 in interest was converted at $0.07 per share into 116,000 shares of common stock.
On January 26, 2018, pursuant to a conversion notice, $30,000 in principal and $1,793 in interest was converted at $0.09 per share into 353,259 shares of common stock.
On January 30, 2018, pursuant to a conversion notice, $40,000 in principal and $2,130 in interest was converted at $0.09 per share into 492,407 shares of common stock.
On February 4, 2018, pursuant to a conversion notice, $22,500 in principal and $2,650 in interest was converted at $0.08 per share into 314,571 shares of common stock.
On February 13, 2018, pursuant to a conversion notice, $20,000 in principal and $1,276 in interest was converted at $0.07 per share into 285,962 shares of common stock.
On February 21, 2018, pursuant to a conversion notice, $40,000 in principal and $4,986 in interest was converted at $0.08 per share into 571,977 shares of common stock.
| F-71 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
On February 23, 2018, pursuant to a conversion notice, $25,000 in principal and $1,173 in interest was converted at $0.07 per share into 351,782 shares of common stock.
On February 23, 2018, pursuant to a conversion notice, $20,000 in principal and $1,320 in interest was converted at $0.07 per share into 296,111 shares of common stock.
On February 28, 2018, pursuant to a conversion notice, $60,000 in principal and $4,027 in interest was converted at $0.06 per share into 1,011,480 shares of common stock.
On March 4, 2018, pursuant to a conversion notice, $40,000 in principal and $5,012 in interest was converted at $0.06 per share into 760,980 shares of common stock.
On March 5, 2018, pursuant to a conversion notice, $28,000 in principal and $1,375 in interest was converted at $0.06 per share into 493,526 shares of common stock.
On March 8, 2018, pursuant to a conversion notice, $27,000 in principal and $1,343 in interest was converted at $0.06 per share into 507,945 shares of common stock.
On March 8, 2018, pursuant to a conversion notice, $50,000 in principal and $3,444 in interest was converted at $0.05 per share into 989,712 shares of common stock.
On March 11, 2018, pursuant to a conversion notice, $60,000 in principal and $7,906 in interest was converted at $0.06 per share into 1,173,828 shares of common stock.
On March 14, 2018, pursuant to a conversion notice, $25,000 in principal and $1,756 in interest was converted at $0.05 per share into 495,473 shares of common stock.
On March 16, 2018, pursuant to a conversion notice, $28,000 in principal and $1,442 in interest was converted at $0.06 per share into 527,637 shares of common stock.
On March 21, 2018, pursuant to a conversion notice, $50,000 in principal and $2,089 in interest was converted at $0.05 per share into 964,609 shares of common stock.
On March 26, 2018, pursuant to a conversion notice, $27,000 in principal and $1,450 in interest was converted at $0.06 per share into 504,251 shares of common stock.
On April 2, 2018, pursuant to a conversion notice, $50,000 in principal and $2,916 in interest was converted at $0.06 per share into 912,659 shares of common stock.
On April 3, 2018, pursuant to a conversion notice, $25,000 in principal and $1,386 in interest was converted at $0.05 per share into 506,649 shares of common stock.
On April 5, 2018, pursuant to a conversion notice, $50,000 in principal and $2,256 in interest was converted at $0.05 per share into 1,088,658 shares of common stock.
On April 11, 2018, pursuant to a conversion notice, $20,000 in principal and $929 in interest was converted at $0.04 per share into 581,358 shares of common stock.
On April 12, 2018, pursuant to a conversion notice, $30,000 in principal and $1,289 in interest was converted at $0.04 per share into 841,095 shares of common stock.
On April 18, 2018, pursuant to a conversion notice, $50,000 in principal and $3,750 in interest was converted at $0.03 per share into 1,560,232 shares of common stock.
| F-72 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
On April 26, 2018, pursuant to a conversion notice, $35,000 in principal and $3,259 in interest was converted at $0.04 per share into 1,062,747 shares of common stock
On April 30, 2018, pursuant to a conversion notice, $25,000 in principal and $526 in interest was converted at $0.04 per share into 686,183 shares of common stock.
On May 14, 2018, pursuant to a conversion notice, $30,000 in principal and $723 in interest was converted at $0.04 per share into 768,274 shares of common stock.
On May 15, 2018, pursuant to a conversion notice, $20,000 in principal and $2,000 in interest was converted at $0.04 per share into 527,577 shares of common stock.
On May 18, 2018, pursuant to a conversion notice, $33,500 in principal and $3,283 in interest was converted at $0.04 per share into 957,891 shares of common stock.
On June 7, 2018, pursuant to a conversion notice, $33,000 in principal and $3,381 in interest was converted at $0.03 per share into 1,045,422 shares of common stock.
On June 8, 2018, pursuant to a conversion notice, $35,000 in principal and $1,035 in interest was converted at $0.04 per share into 1,002,102 shares of common stock.
On June 15, 2018, pursuant to a conversion notice, $32,000 in principal and $3,335 in interest was converted at $0.03 per share into 1,015,377 shares of common stock.
On June 20, 2018, pursuant to a conversion notice, $35,000 in principal and $3,687 in interest was converted at $0.03 per share into 1,111,686 shares of common stock.
On June 21, 2018, pursuant to a conversion notice, $35,000 in principal and $1,135 in interest was converted at $0.04 per share into 1,003,146 shares of common stock.
On June 23, 2018, pursuant to a conversion notice, $20,000 in principal and $2,098 in interest was converted at $0.03 per share into 703,757 shares of common stock.
The Company has 165,657,321 shares reserved for future issuances based on lender requirements at June 30, 2018.
Options:
On April 14, 2016 (“Grant Date”), the board of directors of the Company, approved a grant of 286,000 stock options at an exercise price of $7.50 (market value of the Company’s stock on the Grant Date), to each of the Company’s CEO and a non-executive director. 95,333 of such stock options vested on the Grant Date and expire on April 14, 2021, 95,333 of such stock options vested on April 14, 2017 (first anniversary of the Grant Date) and expire on April 14, 2021 and 95,333 of such stock options vested on April 14, 2018 (second anniversary of the Grant Date) and expire on April 14, 2021. The fair value of each grant of the 286,000 options at Grant Date was $1,962,440 (aggregate total of $3,924,880).
The Company expensed $516,148 and $1,686,444 for these stock option grants during the years ended June 30, 2018 and 2017, respectively. As of June 30, 2018, these options are fully expensed.
| F-73 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
A summary of the Company’s option activity during the years ended June 30, 2018 and 2017 is presented below:
| Weighted | ||||||||
| Number of | Average | |||||||
| Shares | Price Per Share | |||||||
| Outstanding at June 30, 2016 | 572,000 | $ | 7.50 | |||||
| Issued | - | - | ||||||
| Exercised | - | - | ||||||
| Expired | - | - | ||||||
| Outstanding at June 30, 2017 | 572,000 | $ | 7.50 | |||||
| Issued | - | - | ||||||
| Exercised | - | - | ||||||
| Forfeited | - | - | ||||||
| Expired | - | - | ||||||
| Outstanding at June 30, 2018 | 572,000 | $ | 7.50 | |||||
| Exercisable at June 30, 2018 | 572,000 | $ | 7.50 | |||||
| Outstanding and Exercisable: | ||||||||
| Weighted average remaining contractual term | 2.79 | |||||||
| Aggregate intrinsic value | $ | - | ||||||
Warrants:
On July 8, 2016, the 2015 Warrant for 104,762 shares issued to Delafield was fully exercised at a price of $3.00 per share for a total of $314,286 in connection with the July Letter Agreement (See Note 6 – Convertible Notes).
On August 3, 2016, pursuant to the August Letter Agreement, the Company issued 960,000 warrants to purchase shares of the Company’s common stock. 800,000 of these warrants had exercise prices ranging from $3.00 to $5.00 per share and expired five months from the date of issuance. 160,000 of these warrants had an exercise price of $25.00 per share and expired two years from the date of issuance. These warrants were subsequently cancelled as discussed in Note 6 – Convertible Notes.
On August 18, 2016, pursuant to the August Letter Agreement, warrants to purchase 50,000 shares of the Company’s common stock were exercised at a price of $3.00 per share under the first tranche of the Five Month Warrant or $150,000 in the aggregate. These shares were subsequently cancelled and a loss of $37,500 was recorded (See Note 6 – Convertible Notes).
On November 9, 2016, the Company entered into an agreement (the “November Agreement”) to adjust the exercise price of a warrant, issued on September 30, 2013, to purchase 12,000 shares of common stock of the Company. Under the terms of the November Agreement, the exercise price for the shares underlying the warrant was reduced to $3.75 AUD or $2.88 USD per share. The November Agreement did not affect the remaining terms of the warrant. The Company recorded an additional expense of $3,299 AUD related to the repricing.
On December 12, 2016, pursuant to the December Letter Agreement (See Note 6 – Convertible Notes), the Company issued a two-year warrant to purchase 104,000 shares of the Company’s common stock (the “New Warrant”). This warrant has an exercise price of $12.50 per share. This warrant was being treated as a modification of an existing warrant under ASC 718-20-35-3 and the Company determined that since the valuation of the New Warrant does not exceed the value of the 2016 Warrants, the Company will continue to amortize the remainder of the $910,178 value of the 2016 Warrant, which was fully amortized as of February 28, 2017.
As of June 30, 2018, there were 145,517 warrants outstanding and exercisable with expiration dates ranging from September 2018 and through November 2020.
| F-74 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
The following table summarizes warrant activity for the years ended June 30, 2018 and 2017:
| Weighted | ||||||||
| Number of | Average | |||||||
| Shares | Price Per Share | |||||||
| Outstanding at June 30, 2016 | 150,279 | 107.50 | ||||||
| Issued | 1,064,000 | 7.99 | ||||||
| Exercised | (104,762 | ) | 3.00 | |||||
| Forfeited | (960,000 | ) | 7.50 | |||||
| Expired | - | - | ||||||
| Outstanding at June 30, 2017 | 149,517 | $ | 11.25 | |||||
| Issued | - | - | ||||||
| Exercised | - | - | ||||||
| Forfeited | - | - | ||||||
| Expired | (4,000 | ) | - | |||||
| Outstanding at June 30, 2018 | 145,517 | $ | 11.11 | |||||
| Exercisable at June 30, 2018 | 145,517 | $ | 11.11 | |||||
| Outstanding and Exercisable: | ||||||||
| Weighted average remaining contractual term | .76 | |||||||
| Aggregate intrinsic value | $ | - | ||||||
NOTE 9 – COMMITMENTS AND CONTINGENCIES
Legal Matters
From time to time, the Company may be involved in litigation relating to claims arising out of the Company’s operations in the normal course of business. As of June 30, 2018, there were no pending or threatened lawsuits that could reasonably be expected to have a material effect on the results of the Company’s operations.
IRS Liability
As part of its requirement for having a foreign operating subsidiary, the Company is required to file an informational Form 5471 to the Internal Revenue Service (the “IRS”), which is a form that explains the nature of the relationship between the foreign subsidiary and the parent company. From 2012 through the 2014 the Company did not file this form in a timely manner. As a result of the non-timely filings, the Company incurred a penalty from the IRS in the amount of $10,000 per year, or $30,000 in total, plus accrued interest. The Company recorded the penalties for all three years during the year ended June 30, 2017 and is negotiating a payment plan. The Company is current on all subsequent filings.
Operating Agreements
In November 2009, the Company entered into a commercialization agreement with the University of Bath (UK) (the “University”) whereby the Company and the University co-owned the intellectual property relating to the Company’s pro-enzyme formulations. In June 2012, the Company and the University entered into an assignment and amendment whereby the Company assumed full ownership of the intellectual property while agreeing to pay royalties of 2% of net revenues to the University. Additionally, the Company agreed to pay 5% of each and every license agreement subscribed for. The contract is cancellable at any time by either party. To date, no amounts are owed under the agreement.
Operating Leases
On May 4, 2016, the Company entered into a new five-year operating lease agreement with a Horizon Pty Ltd., a related party, of which Mr. Nathanielsz, our CEO, CFO and a director, and his wife are owners and directors, with monthly rent of $3,300 AUD or $2,558 USD, inclusive of GST (See Note 10 – Related Party Transactions).
Future minimum operating lease commitments consisted of the following at June 30, 2018:
| Year Ended June 30, | Amount (USD) | |||
| 2019 | $ | 29,300 | ||
| 2020 | $ | 29,300 | ||
| 2021 | $ | 24,417 | ||
Rent expense for the years ended June 30, 2018 and 2017 was $30,521 and $28,992, respectively.
| F-75 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
Amatsigroup Agreement
The Company entered into a Manufacturing Services Agreement (the “MSA”) and Quality Assurance Agreement (the “QAA”), each with an effective date of August 12, 2016, with Amatsigroup NV (“Amatsigroup”), formerly known as Q-Biologicals, NV, a contract manufacturing organization located in Belgium. Pursuant to the MSA, Amatsigroup produces certain drug substances and products containing certain enzymes for the Company at its facility in Belgium. The Company uses these substances and products for development purposes, including but not limited to future clinical trials. The MSA contemplates payment to Amatsigroup pursuant to a pre-determined fee schedule based on the completion of certain milestones that depend on our manufacturing requirements and final batch yield. The Company anticipates that its payments to Amatsigroup under the MSA will range between $2.5 million and $5.0 million over three years, when the finished drug product is manufactured and released for clinical trials. In the years ended June 30, 2018 and 2017, the Company has incurred $701,973 and $937,219 of costs, respectively under the MSA. The MSA shall continue for a term of three years unless extended by mutual agreement in writing. The Company can terminate the MSA early for any reason upon the required notice period, however, in such event, the pre-payment paid upon signing the MSA is considered non-refundable. Each party to the MSA shall have the right to terminate the MSA by written notice to the other party if the other party commits a material breach of the MSA (subject to a 30-day cure period). The QAA sets forth the parties respective obligations and responsibilities relating to the manufacturing and testing of the products under the MSA. The agreements with Amatsigroup contain certain customary representations, warranties and limitations of liabilities, and confidentiality and indemnity obligations.
Investment Banking Agreement
On February 23, 2018, the Company entered into an agreement with an effective date of February 14, 2018 with a certain investment bank (the “Investment Bank”), pursuant to which the Company retained the Investment Bank as its placement agent. The agreement terminates at the close of business on September 30, 2018. As consideration for such services, the Company shall pay the Investment Bank 8% of the total gross proceeds immediately upon closing a successful capital raise placement. Additionally, the Company shall also pay the Investment Bank non-callable warrants for shares of the Company’s common stock equal to 4% of the proceeds raised. The warrants will have a purchase price equal to 110% of the implied price per share of the placement or 110% of the public market closing price of the Company’s common stock on the date of placement, whichever is lower, and will have an exercise period of five years after the closing of the placement. As of the date of this Annual Report, no funds have been raised pursuant to this agreement.
NOTE 10 – RELATED PARTY TRANSACTIONS
Since its inception, the Company has conducted transactions with directors and director-related entities. These transactions have included the following:
As of June 30, 2018 and June 30, 2017, the Company owed a current and former director a total of $54,753 and $56,802, respectively, for money loaned to the Company throughout the years. The loan balance owed is not interest bearing (See Note 5 – Loans and Note Payable).
As of June 30, 2018 and June 30, 2017, the Company owed its two current directors a total of $32,898 and $35,204, respectively, related to expenses paid on behalf of the Company related to corporate startup costs and intellectual property (See Note 4 – Due to Former Directors – Related Parties).
Effective May 5, 2016, we entered into an agreement for the lease of our principal executive offices with North Horizon Pty Ltd., a related party, of which Mr. Nathanielsz, our CEO, CFO and a director, and his wife are owners and directors. The lease has a five-year term and provides for annual rental payments of $39,600 AUD or $27,302 USD, which includes $3,600 AUD or $2,664 USD of goods and service tax for total payments of $198,000 AUD or $146,500 USD during the term of the lease. As of June 30, 2018, total payments of $112,200 AUD or $83,017 USD remain on the lease.
| F-76 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
Mr. Nathanielsz’s wife, Sylvia Nathanielsz, is and has been a non-executive employee of our Company since October 2015. Mrs. Nathanielsz received an annual salary of $57,675 through January 31, 2018 and is entitled to customary benefits. Effective February 1, 2018, Mrs. Nathanielsz salary was increased, and she now receives an annual salary of $88,788. For the fiscal year ended June 30, 2018, Mrs. Nathanielsz received a total salary of $72,684.
Pursuant to board approval on February 25, 2016, James Nathanielsz shall be paid $4,481 AUD ($3,502 USD), on a monthly basis for the purpose of acquiring and maintaining an automobile. For the year ended June 30, 2018 and 2017, a total of $41,481 and $40,562, respectively, in payments have been made with regards to such automobile allowance. In connection with the payments made pursuant to such automobile allowance, the Company must also pay a fringe benefit tax for the value of the benefit provided. As of June 30, 2018, and 2017, the Company has recorded $14,706 and $13,787, respectively, of additional salary expense related to these tax benefit payments.
Pursuant to the approval of the board of directors, on August 15, 2016, Mr. Nathanielsz was granted a $250,000 bonus for accomplishments achieved while serving as the Company’s chief executive officer during the fiscal year ended June 30, 2016. A total of $130,000 in payments was made in the year ended June 30, 2017. The remaining $120,000 was paid during the year ended June 30, 2018.
Pursuant to the approval of the board of directors, on March 16, 2018, Mr. Nathanielsz was granted a $300,000 AUD ($221,970 USD) bonus for accomplishments achieved while serving as the Company’s chief executive officer during the fiscal years ended June 30, 2017. A total of $59,226 was paid during the year ended June 30, 2018. The balance of the accrued bonus as of June 30, 2018 is $162,744.
NOTE 11 – CONCENTRATIONS AND RISKS
Concentration of Credit Risk
The Company maintains its cash in banks and financial institutions in Australia. Bank deposits in Australian banks are uninsured. The Company has not experienced any losses in such accounts through June 30, 2018.
The company currently primarily relies on funding from two convertible debt lenders. Proceeds received in the year from each of the two lenders were $1,577,018 and $951,900, respectively, which represents approximately 55% and 33%, respectively of total proceeds received by the Company during fiscal year 2018.
Receivable Concentration
As of June 30, 2018 and June 30, 2017, the Company’s receivables were 100% related to reimbursements on GST taxes paid.
Patent and Patent Concentration
The Company has filed six patent applications relating to its lead product, PRP. The Company’s lead patent application has been granted and remains in force in the United States, Europe, Australia, China, Japan, Indonesia, Israel, New Zealand, Singapore, Malaysia, South Africa and Mexico. In Brazil, Canada, Hong Kong, and Republic of Korea, the patent application remains under examination.
In 2016 and early 2017, we filed other patent applications. Three applications were filed under the Patent Cooperation Treaty (the “PCT”). The PCT assists applicants in seeking patent protection by filing one international patent application under the PCT, applicants can simultaneously seek protection for an invention in over 150 countries. Once filed, the application is placed under the control of the national or regional patent offices, as applicable, in what is called the national phase.1 One of the PCT applications filed in November 2016, entered national phase in July 2018 and another PCT application is currently entering national phase in August 2018. A third PCT application is scheduled to enter national phase in October, 2018.
| F-77 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
Further patent applications are expected to be filed to capture and protect additional patentable subject matter based on the Company’s field of technology relating to pharmaceutical compositions of proenzymes for treating cancer.
Foreign Operations
As of June 30, 2018 and June 30, 2017, the Company’s operations are based in Camberwell, Australia, however the majority of research and development is being conducted in the European Union.
On July 22, 2016, the Company formed a wholly owned subsidiary, Propanc (UK) Limited under the laws of England and Wales for the purpose of submitting an orphan drug application with the European Medicines Agency as a small and medium-sized enterprise. As of June 30, 2018, there has been no activity within this entity.
NOTE 12 - DERIVATIVE FINANCIAL INSTRUMENTS AND FAIR VALUE MEASUREMENTS
Derivative Financial Instruments:
The Company applies the provisions of ASC 815-40, Contracts in Entity’s Own Equity, under which convertible instruments and warrants, which contain terms that protect holders from declines in the stock price (reset provisions), may not be exempt from derivative accounting treatment. As a result, warrants and embedded conversion options in convertible debt are recorded as a liability and are revalued at fair value at each reporting date. If the fair value of the warrants exceeds the face value of the related debt, the excess is recorded as change in fair value in operations on the issuance date. The Company had 12,000 warrants and $429,000 of convertible debt, which are treated as derivative instruments outstanding at June 30, 2018.
The Company calculates the estimated fair values of the liabilities for derivative instruments using the Binomial Trees Method. The closing price of the Company’s common stock at June 30, 2018 and 2017 was $0.06 and $0.97, respectively. Volatility, expected remaining term and risk free interest rates used to estimate the fair value of derivative liabilities at June 30, 2018 and 2017, are indicated in the table that follows. The expected term is equal to the remaining term of the warrants and the risk free rate is based upon rates for treasury securities with the same term.
Warrants
| June 30, 2017 | June 30, 2018 | |||||||
| Volatility | 137 | % | 110 | % | ||||
| Expected remaining term | 1.25 | .25 | ||||||
| Risk-free interest rate | 1.24 | % | 1.93 | % | ||||
| Expected dividend yield | None | None | ||||||
Convertible Debt
| Initial Valuations |
June 30, 2017 | June 30, 2018 | |||||||||
| Volatility | 139 - 198 | % | 66-175 | % | 191 - 221 | % | |||||
| Expected remaining term | 1.00 – 2.00 | .21 - 1.63 | .56 - 1.1 | ||||||||
| Risk-free interest rate | 1.33 – 2.31 | % | 1.03 - 1.24 | % | 2.11 - 2.33 | % | |||||
| Expected dividend yield | None | None | None | ||||||||
| F-78 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
Fair Value Measurements:
The Company measures and reports at fair value the liability for derivative instruments. The fair value liabilities for price adjustable warrants and embedded conversion options have been recorded as determined utilizing the Binomial Trees model. The following tables summarize the Company’s financial assets and liabilities measured at fair value on a recurring basis as of June 30, 2018:
| Balance at June 30, 2018 |
Quoted Prices in Active Markets for Identical Assets |
Significant Other Observable Inputs |
Significant Unobservable Inputs |
|||||||||||||
| (Level 1) | (Level 2) | (Level 3) | ||||||||||||||
| Embedded conversion option liabilities | $ | 371,532 | $ | — | $ | — | $ | 371,532 | ||||||||
| Total | $ | 371,532 | $ | — | $ | — | $ | 371,532 | ||||||||
The following tables summarize the Company’s financial assets and liabilities measured at fair value on a recurring basis as of June 30, 2017:
| Balance at June 30, 2017 |
Quoted Prices in Active Markets for Identical Assets |
Significant Other Observable Inputs |
Significant Unobservable Inputs |
|||||||||||||
| (Level 1) | (Level 2) | (Level 3) | ||||||||||||||
| Embedded conversion option liabilities | $ | 877,403 | $ | — | $ | — | $ | 877,403 | ||||||||
| Fair value of liability for warrant derivative instruments | $ | 3,769 | $ | — | $ | — | $ | 3,769 | ||||||||
| Total | $ | 881,172 | $ | — | $ | — | $ | 881,172 | ||||||||
The following is a roll forward for the years ended June 30, 2018 and 2017 of the fair value liability of price adjustable derivative instruments:
| Fair Value of | ||||
| Liability for | ||||
| Derivative | ||||
| Instruments | ||||
| Balance at June 30, 2016 | $ | 1,050,182 | ||
| Effects of foreign currency exchange rate changes | 1,143 | |||
| Initial fair value of embedded conversion option derivative liability recorded as debt discount | 650,000 | |||
| Initial fair value of embedded conversion option derivative liability recorded as change in fair value of embedded conversion option | 214,758 | |||
| Change in fair value included in statements of operations | (1,034,911 | ) | ||
| Balance at June 30, 2017 | 881,172 | |||
| Effects of foreign currency exchange rate changes | 38 | |||
| Reductions due to conversions | (861,695 | ) | ||
| Reductions due to repayment of debt | (199,339 | ) | ||
| Initial fair value of embedded conversion option derivative liability recorded as debt discount | 543,744 | |||
| Initial fair value of embedded conversion option derivative liability recorded as change in fair value of embedded conversion option | 313,694 | |||
| Change in fair value included in statements of operations | (306,082 | ) | ||
| Balance at June 30, 2018 | $ | 371,532 | ||
| F-79 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
NOTE 13 – SUBSEQUENT EVENTS
Conversions
On July 2, 2018, pursuant to a conversion notice, $35,000 in principal and $2,808 in interest was converted at $0.03 per share into 1,262,785 shares of common stock.
On July 6, 2018, pursuant to a conversion notice, $40,000 in principal and $4,570 in interest was converted at $0.03 per share into 1,614,885 shares of common stock.
On July 10, 2018, pursuant to a conversion notice, $25,000 in principal and $2,050 in interest was converted at $0.03 per share into 1,024,621 shares of common stock.
On July 10, 2018, pursuant to a conversion notice, $35,000 in principal and $1,281 in interest was converted at $0.03 per share into 1,329,952 shares of common stock.
On July 13, 2018, pursuant to a conversion notice, $30,000 in principal and $2,480 in interest was converted at $0.02 per share into 1,582,846 shares of common stock.
On July 15, 2018, pursuant to a conversion notice, $30,000 in principal and $3,484 in interest was converted at $0.02 per share into 1,550,185 shares of common stock.
On July 17, 2018, pursuant to a conversion notice, $20,000 in principal and $1,671 in interest was converted at $0.02 per share into 1,128,704 shares of common stock.
On July 22, 2018, pursuant to a conversion notice, $30,000 in principal and $3,529 in interest was converted at $0.02 per share into 2,056,993 shares of common stock.
On July 23, 2018, pursuant to a conversion notice, $30,000 in principal and $1,959 in interest was converted at $0.02 per share into 1,718,250 shares of common stock.
On July 24, 2018, pursuant to a conversion notice, $30,000 in principal and $2,553 in interest was converted at $0.02 per share into 2,170,222 shares of common stock.
On July 25, 2018, pursuant to a conversion notice, $20,000 in principal was converted at $0.02 per share into 1,112,347 shares of common stock.
On July 26, 2018, pursuant to a conversion notice, $20,000 in principal was converted at $0.02 per share into 1,273,885 shares of common stock.
On July 30, 2018, pursuant to a conversion notice, $20,000 in principal was converted at $0.01 per share into 1,388,889 shares of common stock.
On July 31, 2018, pursuant to a conversion notice, $20,000 in principal and $1,733 in interest was converted at $0.01 per share into 1,857,550 shares of common stock.
On July 31, 2018, pursuant to a conversion notice, $15,000 in principal was converted at $0.01 per share into 1,041,667 shares of common stock.
On August 2, 2018, pursuant to a conversion notice, $20,000 in principal and $1,742 in interest was converted at $0.01 per share into 2,119,125 shares of common stock.
| F-80 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
On August 2, 2018, pursuant to a conversion notice, $15,000 in principal was converted at $0.01 per share into 1,260,504 shares of common stock.
On August 3, 2018, pursuant to a conversion notice, $10,000 in principal and $873 in interest was converted at $0.01 per share into 1,184,459 shares of common stock.
On August 3, 2018, pursuant to a conversion notice, $25,000 in principal and $1,693 in interest was converted at $0.01 per share into 2,517,746 shares of common stock.
On August 7, 2018, pursuant to a conversion notice, $20,000 in principal was converted at $0.01 per share into 2,173,913 shares of common stock.
On August 8, 2018, pursuant to a conversion notice, $15,500 in principal and $1,371 in interest was converted at $0.01 per share into 2,162,935 shares of common stock.
On August 8, 2018, pursuant to a conversion notice, $18,000 in principal and $2,195 in interest was converted at $0.01 per share into 3,612,615 shares of common stock.
On August 13, 2018, pursuant to a conversion notice, $20,000 in principal was converted at $0.01 per share into 3,125,000 shares of common stock.
On August 13, 2018, pursuant to a conversion notice, $15,000 in principal and $1,343 in interest was converted at $0.01 per share into 2,867,251 shares of common stock.
On August 13, 2018, pursuant to a conversion notice, $15,000 in principal and $1,049 in interest was converted at $0.01 per share into 2,724,748 shares of common stock.
On August 20, 2018, pursuant to a conversion notice, $12,430 in principal was converted at $0.004 per share into 3,271,053 shares of common stock.
On August 20, 2018, pursuant to a conversion notice, $10,000 in principal and $647 in interest was converted at $0.003 per share into 3,348,009 shares of common stock.
On August 22, 2018, pursuant to a conversion notice, $10,570 in principal and $885 in interest was converted at $0.003 per share into 3,272,857 shares of common stock.
On August 22, 2018, pursuant to a conversion notice, $14,000 in principal and $1,006 in interest was converted at $0.003 per share into 4,566,789 shares of common stock.
On August 24, 2018, pursuant to a conversion notice, $10,000 in principal and $656 in interest was converted at $0.003 per share into 3,860,710 shares of common stock.
On August 27, 2018, pursuant to a conversion notice, $5,235 in interest was converted at $0.003 per share into 1,768,581 shares of common stock.
On August 28, 2018, pursuant to a conversion notice, $10,250 in principal and $750 in interest was converted at $0.002 per share into 4,549,363 shares of common stock.
On August 28, 2018, pursuant to a conversion notice, $8,500 in principal and $565 in interest was converted at $0.002 per share into 3,873,838 shares of common stock.
On August 29, 2018, pursuant to a conversion notice, $8,450 in principal and $563 in interest was converted at $0.002 per share into 3,851,850 shares of common stock.
| F-81 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
On August 31, 2018, pursuant to a conversion notice, $9,000 in principal and $1,159 in interest was converted at $0.003 per share into 3,972,976 shares of common stock.
On September 4, 2018, pursuant to a conversion notice, $3,000 in principal and $237 in interest was converted at $0.002 per share into 1,383,192 shares of common stock.
On September 5, 2018, pursuant to a conversion notice, $3,000 in principal and $237 in interest was converted at $0.002 per share into 1,383,474 shares of common stock.
On September 6, 2018, pursuant to a conversion notice, $7,250 in principal and $545 in interest was converted at $0.002 per share into 3,223,755 shares of common stock.
On September 6, 2018, pursuant to a conversion notice, $28,750 in principal was converted at $0.002 per share into 12,500,000 shares of common stock.
On September 6, 2018, pursuant to a conversion notice, $9,000 in principal and $1,194 in interest was converted at $0.003 per share into 3,986,546 shares of common stock.
On September 7, 2018, pursuant to a conversion notice, $5,000 in principal and $343 in interest was converted at $0.002 per share into 2,283,474 shares of common stock.
On September 11, 2018, pursuant to a conversion notice, $28,500 in principal and $2,174 in interest was converted at $0.02 per share into 1,978,955 shares of common stock.
On September 14, 2018, pursuant to a conversion notice, $50,000 in principal and $4,056 in interest was converted at $0.03 per share into 1,937,475 shares of common stock.
On September 14, 2018, pursuant to a conversion notice, $131,500 in principal and $5,707 in interest was converted at $0.03 per share into 4,759,165 shares of common stock.
June 29, 2018 Securities Purchase Agreement
Effective June 29, 2018, the Company entered into a securities purchase agreement with Coventry Enterprises, LLC (“Coventry Enterprises”), pursuant to which Coventry Enterprises purchased two 8% unsecured convertible promissory notes from the Company in the aggregate principal amount of $200,000, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Coventry Enterprises.
The purchase price of $100,000 of the first note (the “July 2018 Coventry Note”) was paid in cash by Coventry Enterprises on July 2, 2018. After payment of certain legal fees and expenses, net proceeds to the Company from the First Note totaled $95,000. The purchase price of $100,000 of the second note (the “July 2018 Coventry Back-End Note”) was initially paid for by the issuance of an offsetting $100,000 collateralized secured note issued to Company by Coventry Enterprises (the “July 2018 Coventry Enterprises Note”). The terms of the July 2018 Coventry Back-End Note require cash funding prior to any conversion thereunder. The July 2018 Coventry Back-End Note is due February 29, 2019, unless certain conditions are not met, in which case both the July 2018 Coventry Back-End Note and the July 2018 Coventry Enterprise Note may both be cancelled
The maturity date of the July 2018 Coventry Note and the July 2018 Coventry Back-End Note is June 29, 2019. The outstanding principal amounts plus accrued interest under both the July 2018 Coventry Note and the July 2018 Coventry Back-End Note are convertible into shares of common stock of the Company at a conversion price equal to 61% of the lowest closing bid price of the Company’s common stock as reported on the exchange or quotation system on which the Company’s shares are then traded for the ten prior trading days including the day upon which a notice of conversion is received by the Company from Coventry Enterprises. Coventry Enterprises shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Coventry Enterprises and its affiliates, exceeds 9.9% of the outstanding shares of the Company’s common stock.
| F-82 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
The July 2018 Coventry Note may be prepaid by the Company with certain penalties until 180 days from the issuance date. The July 2018 Coventry Back-End Note may not be prepaid by the Company.
Upon an event of default, principal and accrued interest will become immediately due and payable under the notes. Additionally, upon an event of default, both notes will accrue interest at a default interest rate of 24% per annum or the highest rate of interest permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
July 13, 2018 Securities Purchase Agreement
Effective July 13, 2018, the Company entered into a securities purchase agreement with Eagle Equities, pursuant to which Eagle Equities purchased a convertible promissory note (the “July 2018 Note”) from the Company in the aggregate principal amount of $75,000, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Eagle Equities any time after the six month anniversary of the July 2018 Eagle Note. The transaction closed on July 16, 2018 and on July 19, 2018 the Company received proceeds of $71,250 as $3,750 was paid directly to legal fees.
The maturity date of the July 2018 Eagle Note is July 13, 2019. The July 2018 Eagle Note bears interest at a rate of 8% per annum, which interest shall be paid by the Company to Eagle Equities in shares of the Company’s common stock upon receipt of a notice of conversion by the Company from Eagle Equities at any time after the six-month anniversary of the Note.
Additionally, Eagle Equities has the option to convert all or any amount of the principal face amount of the July 2018 Eagle Note, at any time, for shares of the Company’s common stock at a price equal to 60% of the lowest closing bid price of the Company’s common stock for the ten prior trading days, including the day upon which the Company receives a notice of conversion, subject to adjustment in certain events. Eagle Equities shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Eagle Equities and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock.
The July 2018 Eagle Note may be prepaid by the Company with certain penalties until January 9, 2019.
Upon an event of default, principal and accrued interest will become immediately due and payable under the notes. Additionally, upon an event of default, both notes will accrue interest at a default interest rate of 24% per annum or the highest rate of interest permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
August 28, 2018 Securities Purchase Agreement
Effective August 28, 2018, the Company entered into a securities purchase agreement (the “Power Up Purchase Agreement”) with Power Up Lending Group Ltd. (“Power Up”), pursuant to which Power Up purchased a convertible promissory note (the “August 28, 2018 Power Up Note”) from the Company in the aggregate principal amount of $53,000, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Power Up. The transactions contemplated by the Power Up Purchase Agreement closed on August 28, 2018.
The maturity date of the August 28, 2018 Power Up Note is August 28, 2019 (the “Maturity Date”). The August 28, 2018 Power Up Note bears interest at a rate of 8% per annum, which interest may be paid by the Company to Power Up in shares of the Company’s common stock, but shall not be payable until the August 2018 Power Up Note becomes payable, whether at the Maturity Date or upon acceleration or by prepayment, as described below.
Additionally, Power Up has the option to convert all or any amount of the principal face amount of the August 28, 2018 Power Up Note, starting on February 24, 2019.
The August 28, 2018 Power Up Note may be prepaid by the Company until 180 days from the issuance date.
| F-83 |
PROPANC BIOPHARMA, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
Upon an event of default, interest on the outstanding principal shall accrue at a default interest rate of 22% per annum. In the event that the Company fails to deliver to Power Up shares of common stock issuable upon conversion of principal or interest under the August 28, 2018 Power Up Note within three business days of a notice of conversion by Power Up, the Company shall incur a penalty of $500, provided, however, that such fee shall not be due if the failure to deliver the shares is a result of a third party such as the transfer agent.
August 29, 2018 Securities Purchase Agreement
Effective August 29, 2018, the Company entered into a securities purchase agreement with Eagle Equities, pursuant to which Eagle Equities purchased a convertible promissory note (the “August 29, 2018 Eagle Note”) from the Company in the aggregate principal amount of $105,000, such principal and the interest thereon convertible into shares of the Company’s common stock at the option of Eagle Equities any time after the six month anniversary of the August 29, 2018 Eagle Note. The transactions contemplated by the agreement closed on August 30, 2018.
The maturity date of the August 29, 2018 Eagle Note is August 29, 2019. The August 2018 Eagle Note bears interest at a rate of 8% per annum, which interest shall be paid by the Company to Eagle Equities in shares of the Company’s common stock upon receipt of a notice of conversion by the Company from Eagle Equities at any time after the six month anniversary of the August 29, 2018 Eagle Note.
Additionally, Eagle Equities has the option to convert all or any amount of the principal face amount of the August 29, 2018 Eagle Note, at any time, into shares of the Company’s common stock at a price equal to 60% of the lowest closing bid price (the “Closing Bid Price”) of the Company’s common stock as reported on the OTC Markets quotation system for the ten prior trading days, including the day upon which the Company receives a notice of conversion from Eagle Equities (the “Conversion Price”). However, in the event that the Company’s common stock is restricted by the DTC for any reason, the Conversion Price shall be lowered to 50% of the lowest Closing Bid Price for the duration of such restriction. If the Company fails to maintain a reserve of shares of its common stock at least four times the number of shares issuable upon conversion of the August 28, 2018 Eagle Note for at least 60 days after the issuance of the August 28, 2018 Eagle Note, the conversion discount shall be increased by 10%. Notwithstanding the foregoing, Eagle Equities shall be restricted from effecting a conversion if such conversion, along with other shares of the Company’s common stock beneficially owned by Eagle Equities and its affiliates, exceeds 4.99% of the outstanding shares of the Company’s common stock.
The August 29, 2018 Eagle Note may be prepaid by the Company until February 25, 2019.
Upon an event of default, interest on the outstanding principal shall accrue at a default interest rate of 24% per annum or at the highest rate permitted by law. Further, certain events of default may trigger penalty and liquidated damage provisions.
Pre-Payment of March 5, 2018 Power Up Note
On August 28, 2018, the Company prepaid the outstanding principal balance of $53,000 and related accrued interest of $2,033 that was due under the March 5, 2018 Power Up Note. The Company incurred a penalty in the amount of $20,362 as a result of the pre-payment.
Payment of Accrued Bonus Award
On August 29, 2018, the Company made a $20,000 AUD ($17,110 USD) payment to James Nathanielsz related to the cash bonus that was approved on March 16, 2018 (see Note 10 – Related Party Transactions).
On September 11, 2018, the Company made a $20,000 AUD ($17,246 USD) payment to James Nathanielsz related to the cash bonus that was approved on March 16, 2018 (see Note 10 – Related Party Transactions).
Exercise of September 30, 2013 Warrant
On August 29, 2018, the Company received payment of $39.24 AUD for the exercise of a warrant for 12,000 shares of the Company’s common stock.
September 13, 2018 Collaboration Agreement
On September 13, 2018, the Company entered into a two-year collaboration agreement with the University of Jaen (the “University”) to provide certain research services to the Company. In consideration of such services, the Company has agreed to pay the University approximately 52,000 Euros ($60,762 USD) in year one and a maximum of 40,000 Euros ($46,740 USD) in year two. Additionally, in exchange for full ownership of the intellectual property the Company has agreed to pay royalties of 2% of net revenues to the University.
| F-84 |
Subject to Completion, dated February 25, 2019
Prospectus
111,203,954 Shares

Propanc Biopharma, Inc.
Common Stock
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION.
The following table sets forth the costs and expenses payable by us in connection with the issuance and distribution of the securities being registered hereunder. The selling stockholder will bear no expenses associated with this offering except for any broker discounts and commissions or equivalent expenses and expenses of the selling stockholder’s legal counsel applicable to the sale of its shares. All of the amounts shown are estimates, except for the U.S. Securities and Exchange Commission registration fee.
| Item | Amount to be paid | |||
| SEC registration fee | $ | 192.60 | ||
| Legal fees and expenses | 20,000 | |||
| Accounting fees and expenses | 5,000 | |||
| Miscellaneous fees and expenses | 500 | |||
| Total | $ | 25,692.60 | ||
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
Our Certificate of Incorporation contains provisions that limit the liability of our directors for monetary damages to the fullest extent permitted by Delaware law. Consequently, our directors will not be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duties as directors, except liability for:
| ● | any breach of the director’s duty of loyalty to us or our stockholders; | |
| ● | any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; | |
| ● | unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law; or | |
| ● | any transaction from which the director derived an improper personal benefit. |
Our Certificate of Incorporation provides that we are required to indemnify our directors and officers, in each case to the fullest extent permitted by Delaware law. Our Certificate of Incorporation also provides that we are obligated to advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding, and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in that capacity regardless of whether we would otherwise be permitted to indemnify him or her under the provisions of Delaware law.
The limitation of liability and indemnification provisions in our Certificate of Incorporation may discourage stockholders from bringing a lawsuit against our directors and officers for breach of their fiduciary duty. They may also reduce the likelihood of derivative litigation against our directors and officers, even though an action, if successful, might benefit us and other stockholders. Further, a stockholder’s investment may be adversely affected to the extent that we pay the costs of settlement and damage awards against directors and officers as required by these indemnification provisions
In addition, in the future, we intend to enter into indemnification agreements with our directors and officers and some of our executives may have certain indemnification rights arising under their employment agreements with us. These indemnification agreements may require us, among other things, to indemnify our directors and officers for some expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by a director or officer in any action or proceeding arising out of his or her service as one of our directors or officers, or any of our subsidiaries or any other company or enterprise to which the person provides services at our request.
The above discussion of our Certificate of Incorporation and Delaware law is not intended to be exhaustive and is respectively qualified in its entirety by such bylaws and applicable Delaware law.
These indemnification provisions and the indemnification agreements may be sufficiently broad to permit indemnification of our officers and directors for liabilities, including reimbursement of expenses incurred, arising under the Securities Act. We have been advised that, in the opinion of the SEC, indemnification of directors or officers for liabilities arising under the Securities Act is against public policy and, therefore, such indemnification provisions may be unenforceable.
We maintain a general liability insurance policy that covers certain liabilities of directors and officers of our corporation arising out of claims based on acts or omissions in their capacities as directors or officers.
| 80 |
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES
Shares Issued for Services
On June 5, 2015, we issued 27,033 shares of our common stock to a consultant pursuant to a consulting agreement, dated May 7, 2015.
On June 16, 2016, we issued 25,000 shares of our common stock to a consultant pursuant to a consulting agreement, dated April 22, 2016. In addition, the consulting agreement allowed for 10,000 shares of our common stock to be issued for certain reports and another 5,000 shares of our common stock to be issued for specified consulting services. On June 16, 2016, such 15,000 shares of our common stock were issued pursuant to the consulting agreement.
On July 14, 2016, we entered into an addendum to two consulting agreements entered referenced above. We then owed the consultant $60,000 related to the May 7, 2015 agreement for monthly consulting fees and $100,000 related to the April 22, 2016 agreement, which was comprised of a $10,000 retainer and $90,000 for three reports issued by the consultant. Pursuant to such addendum, we agreed to issue 24,000 shares of our common stock in consideration of the $60,000 in outstanding fees and 24,000 shares of our common stock in forgiveness of future monthly consulting fees, valued at $95,400. In addition, we agreed to issue 40,000 shares of our common stock in consideration for the $100,000 in outstanding fees. The shares were issued on November 4, 2016.
On June 29, 2017, the above May 7, 2015 consulting agreement was further amended, and we agreed to issue the consultant 100,000 shares of our common stock as consideration to eliminate the monthly retainer fee of $7,500 on a going forward basis and to waive the consultant’s right of first refusal to act as lead book running manager of any of our public or private offering of securities or any other financing during the term of the engagement. The 100,000 shares were issued on July 25, 2017.
On October 1, 2015, we entered into an agreement with a consultant to provide services to us over a one-year period. We agreed to issue the consultant 6,000 shares of our common stock and an additional 6,000 shares of our common stock on April 1, 2016 unless we terminated the agreement before then. On October 1, 2015, we issued 4,400 and 1,600 shares of our common stock to the consultant related to this agreement. In February 2016, we terminated this agreement.
On November 1, 2015, we entered into a consulting agreement with a consultant to provide services to us over a nine-month period. We agreed to issue the consultant 8,480 shares of our common stock. On August 8, 2016, we issued such 8,480 shares to the consultant.
On December 30, 2015, we entered into a consulting agreement with a consultant to provide services to us over a nine-month period. We agreed to issue the consultant 4,000 shares of our common stock. On January 4, 2016, we issued such 4,000 shares to the consultant.
On January 31, 2016, we entered into a consulting agreement with a consultant to provide services to us over a five-month period in exchange for 36,000 shares of our common stock. On August 23, 2016, we issued such 36,000 shares to the consultant.
On October 27, 2016, we entered into an agreement with a third party for professional services to us over a six-month period commencing on October 10, 2016 in exchange for a monthly fee of $22,500, of which $10,000 a month was to be paid in cash and $12,500 per month in shares of our common stock. Additionally, we acknowledged an existing outstanding balance due of $20,500 for September 2016 services provided by such party to us. On March 2, 2017, we issued 30,000 shares of our common stock to such third party as consideration for the $75,000 of consulting expenses provided to us.
On February 1, 2017, we received an invoice for $30,000 from a third party for six months of consulting services performed during the period of August 1, 2016 through January 31, 2017. The invoice was payable 50% in cash and 50% in shares of our common stock. We issued 30,000 shares on July 25, 2017 as the share consideration due under such agreement.
On May 10, 2017, we entered into a seven-month agreement with a third party for growth strategy consulting services to be provided to us, pursuant to which we agreed to issue 7,500 shares of our common stock per month to such third party as consideration for the services. In June of 2018, the shares were determined by our board of directors to be non-issuable due to non-performance and such shares were not issued under the agreement.
On August 1, 2017, we received an invoice for $30,000 from a third party for six months of consulting services provided to us during the period of February 1, 2017 through July 31, 2017. The invoice was payable in shares of our common stock. On February 15, 2018, we issued 234,375 shares of our common stock to such consultant as payment for the services provided.
| 81 |
On December 29, 2017, we entered into a one-year consulting agreement with a consultant for certain consulting, advisory and media services to be provided to us. As compensation for such services, among other consideration, we agreed to pay the consultant an upfront fee of 500,000 shares of our common stock, with up to 750,000 additional shares to be issued on the six-month anniversary of the date of the consulting agreement at our sole discretion. On February 15, 2018, we issued such 500,000 shares to the consultant.
On February 1, 2018, we received an invoice for $30,000 from a third party for six months of consulting services provided to us during the period of August 1, 2017 through January 31, 2018. The invoice was payable in shares of our common stock. We issued 234,375 shares of our common stock on February 15, 2018 as payment for such services.
On November 28, 2018, we issued 1,000,000 shares of our common stock, valued at $30,000, in connection with certain legal services provided to us.
On December 6, 2018, we entered into an agreement with a certain consultant to provide services over a six-month period beginning November 1, 2018 and ending May 1, 2019 in exchange for 2,000,000 shares of our common stock. On December 27, 2018, we issued such 2,000,000 shares, valued at $39,000.
Settlement of Accounts Payable for Shares of Common Stock
On February 13, 2017, we entered into an agreement with a third party whereby we agreed to issue and deliver to the third party, in lieu of payment of $50,000 of existing accounts payable, shares of our common stock. On March 2, 2017, we issued 16,667 shares of its common stock at a per share price of $3.00 in consideration for the $50,000 in accounts payable.
Issuance of Shares of Common Stock upon Conversion
Fiscal Year 2016
During our first quarter ended September 30, 2015, we issued 32,090 shares of our common stock at an average conversion price of $4.89 as a result of the conversion of principal and interest in the aggregate amount of $155,760 underlying certain convertible notes converted during such period.
During our second quarter ended December 31, 2015, we issued 250,381 shares of our common stock at an average conversion price of $4.34 as a result of the conversion of principal and interest in the aggregate amount of $970,322 underlying certain convertible notes converted during such period.
During our third quarter ended March 31, 2016, we issued 657,537 shares of our common stock at an average conversion price of $2.96as a result of the conversion of principal and interest in the aggregate amount of $1,656,749 underlying certain notes converted during such period.
During our fourth quarter ended June 30, 2016, we issued 454,649 shares of our common stock at an average conversion price of $4.32 as a result of the conversion of principal and interest in the aggregate amount of $2,116,413 underlying certain convertible notes converted during such period.
Fiscal Year 2017
During our first quarter ended September 30, 2016, we issued 61,992 shares of our common stock at an average conversion price of $2.48 as a result of the conversion of principal and interest in the aggregate amount of $153,610 underlying certain convertible notes converted during such period.
During our second quarter ended December 31, 2016, we issued 255,428 shares of our common stock at an average conversion price of $1.73 as a result of the conversion of principal and interest in the aggregate amount of $424,374 underlying certain convertible notes converted during such period.
During our third quarter ended March 31, 2017, we issued 228,301 shares of our common stock at an average conversion price of $1.52 as a result of the conversion of principal and interest in the aggregate amount of $356,257 underlying certain convertible notes converted during such period.
During our fourth quarter ended June 30, 2017, we issued 689,189 shares of our common stock at an average conversion price of $0.69 as a result of the conversion of principal and interest in the aggregate amount of $472,495 underlying certain convertible notes converted during such period.
| 82 |
Fiscal Year 2018
During our first quarter ended September 30, 2017, we issued 1,767,902 shares of our common stock at an average conversion price of $0.27 as a result of the conversion of principal and interest in the aggregate amount of $432,791 underlying certain convertible notes converted during such period.
During our second quarter ended December 31, 2017, we issued 11,332,098 shares of our common stock at an average conversion price of $0.09 as a result of the conversion of principal and interest in the aggregate amount of $945,137 underlying certain convertible notes converted during such period.
During our third quarter ended March 31, 2018, we issued 12,422,576 shares of our common stock at an average conversion price of $0.07 as a result of the conversion of principal and interest in the aggregate amount of $796,772 underlying certain convertible notes converted during such period.
During our fourth quarter ended June 30, 2018, we issued 15,374,813 shares of our common stock at an average conversion price of $0.04 as a result of the conversion of principal and interest in the aggregate amount of $595,488 underlying certain convertible notes converted during such period.
Fiscal Year 2019
During our first quarter ended September 30, 2018, we issued 129,142,548 shares of our common stock at an average conversion price of $0.01 as a result of the conversion of principal and interest in the aggregate amount of $1,413,317 underlying certain convertible notes converted during such period.
During our second quarter ended December 31, 2018, we issued 63,842,216 shares of our common stock at an average conversion price of $0.02 as a result of the conversion of principal and interest in the aggregate amount of $1,095,100 underlying certain outstanding convertible notes converted during such period.
We had 806,210,142 shares of our common stock reserved for future issuances based on lender note conversion requirements pursuant to underlying financing documents at December 31, 2018.
Other Issuance of Shares of Common Stock
On October 5, 2018, we issued 3,850,597 shares of our common stock to L2 Capital as the commitment fee under the L2 Equity Purchase Agreement.
Issuance of Options
On April 14, 2016, our board of directors approved a grant of 286,000 stock options, with an exercise price of $7.50 (market value of the shares of our common stock on such grant date), to each of Mr. Nathanielsz, our Chief Executive Officer, Chief Financial Officer and a director, and Dr. Kenyon, our non-executive director. 95,333 of such options vested on such grant date and expire on April 14, 2021, 95,333 of such options vested on April 14, 2017 (first anniversary of such grant date) and expire on April 14, 2021 and 95,333 of such options vested on April 14, 2018 (second anniversary of such grant date) and expire on April 14, 2021. The fair value of each grant of the 286,000 options at such grant date was $1,962,440 (aggregate total of $3,924,880).
Issuance of Warrants
In connection with the consulting agreement, dated May 7, 2015, we issued to a consultant 5-year warrants to purchase 13,517 shares of our common with an exercise price of $7.50 per share.
In connection with the consulting agreement, dated May 21, 2015, we issued to a consultant 5-year warrants to purchase 4,000 shares of our common stock with an exercise price of $17.50 per share.
On October 28, 2015, pursuant to a convertible debenture, we issued 4-year warrants to purchase 104,762 shares of our common stock with an exercise price of $150 per share.
In connection with the consulting agreement, dated November 11, 2015, on February 22, 2016, we issued to a consultant 5-year warrants to purchase 16,000 shares of our common stock with an exercise price of $11.25 per share.
On July 8, 2016, the 2015 Warrant to purchase 104,762 shares of our common stock issued to Delafield Limited Investments (“Delafield”) was fully exercised at a price of $3.00 per share for a total of $314,286 in connection with the Letter Agreement, dated July 1, 2016, entered into with Delafield.
| 83 |
On August 3, 2016, pursuant to the Letter Agreement, dated August 3, 2016 (the “August Letter Agreement”), entered into with Delafield we issued warrants to purchase 960,000 shares of our common stock. 800,000 of these warrants had exercise prices ranging from $3.00 to $5.00 per share and expired five months from the date of issuance. 160,000 of these warrants had an exercise price of $25.00 per share and expired two years from the date of issuance. These warrants were subsequently cancelled.
On August 18, 2016, pursuant to the August Letter Agreement, warrants to purchase 50,000 shares of our common stock issued to Delafield were exercised at a price of $3.00 per share under the first tranche of the Five Month Warrant or $150,000 in the aggregate. These shares were subsequently cancelled.
On November 9, 2016, we entered into an agreement (the “November Agreement”) to adjust the exercise price of a warrant, issued on September 30, 2013, to purchase 12,000 shares of our common stock. Under the terms of the November Agreement, the exercise price for the shares underlying the warrant was reduced to $2.88 per share. The November Agreement did not affect the remaining terms of the warrant.
On December 12, 2016, pursuant to the December Letter Agreement, dated December 2, 2016, entered into with Delafield, we issued a 2-year warrant to purchase 104,000 shares of our common stock with an exercise price of $12.50 per share.
As of December 31, 2018, there were 29,517 warrants outstanding and exercisable with expiration dates commencing May 2020 and continuing through November 2020, with a weighted average exercise price per share of $9.53. Except as otherwise noted, the securities in these transactions were sold in reliance on the exemption from registration provided in Section 4(a)(2) of the Securities Act for transactions not involving any public offering. Each of the persons acquiring the foregoing securities was an accredited investor (as defined in Rule 501(a) of Regulation D) and confirmed the foregoing and acknowledged, in writing, that the securities must be acquired and held for investment. All certificates evidencing the shares sold bore a restrictive legend. No underwriter participated in the offer and sale of these securities, and no commission or other remuneration was paid or given directly or indirectly in connection therewith. The proceeds from these sales were used for general corporate purposes.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits.
| 84 |
| 85 |
| 86 |
| 87 |
| 88 |
* Filed herewith.
† Management contract or compensatory plan or arrangement.
(b) Financial Statement Schedules.
All financial statement schedules are omitted because the information called for is not required or is shown either in the financial statements or in the notes thereto.
Item 17. Undertakings.
(a) The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
| 89 |
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof;
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering;
(5) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(6) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i.) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii.) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii.) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv.) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue.
| 90 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Melbourne, in the State of Victoria, Australia, on February 25, 2019.
| PROPANC BIOPHARMA, INC. | ||
| By: | /s/ James Nathanielsz | |
| James Nathanielsz | ||
Chief Executive Officer and Chief Financial Officer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | ||
KNOW ALL BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints James Nathanielsz as his true and lawful attorney-in-fact and agent, with the full power of substitution, for him and in his name, place or stead, in any and all capacities, to sign any and all amendments to this registration statement (including post-effective amendments), and to sign any registration statement for the same offering covered by this registration statement that is to be effective upon filing pursuant to Rule 462 promulgated under the Securities Act, and all post-effective amendments thereto, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agents or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ James Nathanielsz | Chief Executive Officer, Chief Financial | February 25, 2019 | ||
| James Nathanielsz | Officer, Active Chairman and a Director | |||
| /s/ Julian Kenyon | Director | February 25, 2019 | ||
| Julian Kenyon |
| 91 |