UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
| PROPANC HEALTH GROUP |
| CORPORATION |
| (Exact name of registrant as specified in its |
| charter) |
| Delaware | 2834 | 33-0662986 | ||
| (State or other jurisdiction of | (Primary Standard Industrial | (I.R.S. Employer | ||
| incorporation) | Classification Code Number) | Identification No.) |
Level 2, 555 Riversdale Road
Camberwell, VIC, 3124 Australia
Tel. No.: +61 (0)3 9882 0780
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Copies to:
Gregg Jaclin, Esq.
Szaferman, Lakind, Blumstein & Blader, PC
101 Grovers Mill Road
Second Floor
Lawrenceville, NJ 08648
(609) 275-0400
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: þ
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | ¨ |
| Non-accelerated filer | ¨ | Smaller reporting company | þ |
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered | Amount to be Registered | Proposed Maximum Offering Price Per Share (2) | Proposed Maximum Aggregate Offering Price (2) | Amount of Registration Fee | ||||||||||||
| Common stock, par value $0.001 par value per share (the “Common Stock”), underlying convertible notes (1) | 171,000,000 | $ | 0.03 | $ | 5,130,000 | $ | 516.59 | |||||||||
| Total | 171,000,000 | $ | 0.03 | $ | 5,130,000 | $ | 516.59 | |||||||||
(1) This registration statement covers the resale by our selling shareholders of up to 171,000,000 shares of Common Stock previously issued to such selling shareholders.
(2) The offering price has been estimated solely for the purpose of computing the amount of the registration fee in accordance with Rule 457(c) and Rule 457(o) of the Securities Act on the basis of the closing bid price of the Common Stock of the registrant as reported on the OTCQB Marketplace on March 17, 2016.
THE REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON SUCH DATE OR DATES AS MAY BE NECESSARY TO DELAY ITS EFFECTIVE DATE UNTIL THE REGISTRANT SHALL FILE A FURTHER AMENDMENT WHICH SPECIFICALLY STATES THAT THIS REGISTRATION STATEMENT SHALL THEREAFTER BECOME EFFECTIVE IN ACCORDANCE WITH SECTION 8(a) OF THE SECURITIES ACT OR UNTIL THE REGISTRATION STATEMENT SHALL BECOME EFFECTIVE ON SUCH DATE AS THE COMMISSION, ACTING PURSUANT TO SUCH SECTION 8(a), MAY DETERMINE.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission becomes effective. This preliminary prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | Subject to completion, dated March 24, 2016 |
PROPANC HEALTH GROUP CORPORATION
PROSPECTUS
171,000,000 Shares of Common Stock
Propanc Health Group Corporation (the “Company”) closed a financing transaction by entering into a Securities Purchase Agreement dated October 28, 2015 (the “Purchase Agreement”) with Delafield Investment Limited (the “Purchaser”) for an aggregate subscription amount of $4,400,000 (purchase price of $4,000,000 with a 10% original issue discount) (the “October 2015 Financing”). Pursuant to the Purchase Agreement, the Company issued the following to the Purchaser: (i) 5% Original Issue Discount Senior Secured Convertible Debenture with an aggregate principal amount of $4,400,000 (the “Debenture”) and (ii) Common Stock Purchase Warrant to purchase an aggregate of 26,190,476 shares of the Company’s Common Stock for an exercise price of $0.60 per share for a period of four (4) years from the effective date of the registration statement (the “Warrant”).
In connection with the execution of the Purchase Agreement and the October Financing, the Company and the Purchaser, as applicable also entered into the Debenture, the Warrant, that certain Registration Rights Agreement and that certain Security Agreement, each dated as of October 28, 2015, and the Deposit Control Account Agreement, dated November 12, 2015 (all such documents along with the Purchase Agreement, the “October Financing Documents”).
On March 11, 2016, the Company and the Purchaser entered into an Addendum (the “Addendum”) pursuant to which the Company and the Purchaser agreed to certain new terms with respect to the October 2015 Financing. However, the aggregate principal amount of the Debenture and the terms of the Warrant were not modified.
The Company filed a registration statement on Form S-1 on November 23, 2015, which was deemed effective on December 10, 2015, pursuant to which the Company previously registered an aggregate of 98,404,985 shares of the Company's Common Stock consisting of: (i) 72,214,509 shares underlying the Debenture; and (ii) 26,190,476 shares of Common Stock issuable upon exercise of the Warrant (the “Prior Registration Statement”).
Under the terms of the Debenture, the Company was entitled to a reduction in the principal amount of the October 2015 Financing of (i) $25,000 upon the Company’s filing on the Prior Registration Statement within the time period specified and (ii) $25,000 upon the effectiveness of the Prior Registration Statement within the time period specified. Both of these conditions were met resulting in a $50,000 reduction of the principal amount of the October 2015 Financing such that the aggregate principal amount is $4,350,000 as of the date of this Registration Statement.
This prospectus is to be used by certain funds and accounts (the “Selling Security Holders”) in connection with a potential resale by certain Seller Security Holders of up to an aggregate of 171,000,000 shares of the Company's Common Stock, which underlies the Debenture.
The selling shareholders named in this prospectus are offering all of the shares of common stock offered through this prospectus. The common stock to be sold by the selling shareholders as provided in the “Selling Shareholders” section is shares of our common stock, par value $0.001 per share (the “Common Stock”), that have already been issued and are currently outstanding. We will not receive any proceeds from the sale of the Common Stock covered by this prospectus.
Our Common Stock is traded on OTCQB; an OTC market tier for companies that report to the SEC. Investors can find quotes and market information for the Company at www.otcmarkets.com under the ticker symbol “PPCH”. The Selling Security Holders have not engaged any underwriter in connection with the sale of their shares of Common Stock.
Common Stock being registered in this registration statement may be sold by Selling Security Holders at prevailing market prices or privately negotiated prices or in transactions that are not in the public market. On March 17, 2016, the closing price of our Common Stock was $0.03 per share.
We are an emerging growth company as that term is used in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”) and are subject to reduced public company reporting requirements.
Investing in our Common Stock involves a high degree of risk. Before buying any shares, you should carefully read the discussion of material risks of investing in our Common Stock in “Risk Factors” beginning on page 6 of this prospectus.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is March 24, 2016
TABLE OF CONTENTS
Please read this prospectus carefully. It describes our business, our financial condition and results of operations. We have prepared this prospectus so that you will have the information necessary to make an informed investment decision.
You should rely only on information contained in this prospectus. We have not authorized any other person to provide you with different information. This prospectus is not an offer to sell, nor is it seeking an offer to buy, these securities in any state where the offer or sale is not permitted. The information in this prospectus is complete and accurate as of the date on the front cover, but the information may have changed since that date.
This summary highlights selected information contained elsewhere in this prospectus. This summary does not contain all the information that you should consider before investing in the Common Stock. You should carefully read the entire prospectus, including “Risk Factors”, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the Financial Statements, before making an investment decision. In this Prospectus, the terms “Propanc,” “Propanc Health,” “Propanc Health Group,” “Company,” “we,” “us” and “our” refer to Propanc Health Group Corporation or any of its subsidiaries.
Overview
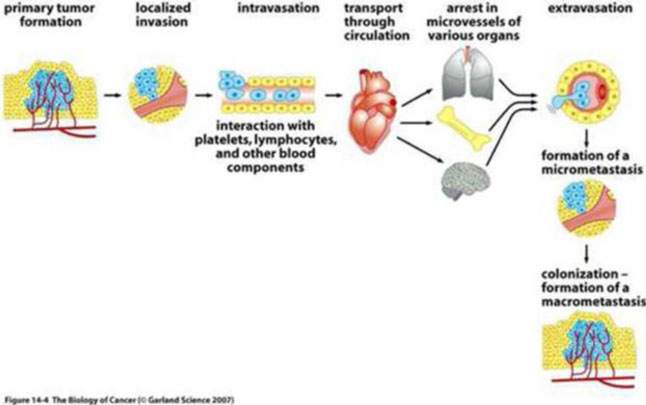
We are a development stage healthcare company that is currently focused on developing new cancer treatments for patients, suffering from pancreatic, ovarian and colorectal cancers. Together with our scientific and oncology consultants, we have developed a rational, composite formulation of anti-cancer compounds, which together exert a number of effects designed to control or prevent tumors from recurring and spreading through the body. Our leading products are variations upon our novel formulation and involve or employ pro-enzymes, which are inactive precursors of enzymes. As a result of positive early indications of the anti-cancer effects of our technology, we intend to submit our pro-enzyme treatment to the rigorous, formal non-clinical and clinical development and trial processes required to obtain the regulatory approval necessary to commercialize it and any product(s) derived and/or to be derived therefrom.
In the near term, we intend to target patients with limited remaining therapeutic options for the treatment of solid tumors such as colorectal, ovarian or pancreatic tumors. In the future, we intend to development our lead product to treat (i) early stage cancer and (ii) pre-cancerous diseases and (iii) as a preventative measure for patients at risk of developing cancer based on genetic screening.
Key Highlights of this opportunity are:
| · | Potential cancer treatment: a once-daily pro-enzyme treatment as a clinically proven therapeutic option in cancer treatment and prevention. |
| · | Multiple mechanisms of action on cancerous or carcinogenic cells: our treatment exerts multiple effects on cancerous cells which inhibits tumor growth and potentially stop the tumor from spreading through the body in contrast to cancer treatments currently available that lack sufficient efficacy to achieve a durable clinical response by preventing tumor recurrence, or inhibiting new growths which spread through the body. As we progress our research, we intend to elucidate further the multiple mechanisms of action to identify opportunities to expand our intellectual property portfolio. Furthermore, we hope to uncover the molecular targets of the pro-enzymes to identify potential opportunities for developing new compounds. |
1
| · | Encouraging data from patient treatment: Scientific research undertaken over the last 15 years and clinical experience from treating patients in the United Kingdom (the “UK”) and Australia has provided evidence that PRP may be an effective treatment against cancer and warrants further development. |
| · | Unique intellectual property: We are focusing on building a significant portfolio of intellectual property around the use of pro-enzymes in the treatment of cancer, identifying new formulations, alternative routes of administration and potential new therapeutic targets. The PRP drug product is an enhanced pro-enzyme formulation comprising amylase and pro-enzymes of trypsinogen and chymotrypsinogen, in a specific ratio which synergistically enhances the anti-cancer effects of the pro-enzymes compared to when used as singular entities. Patent protection is currently being sought for this PRP drug product, which forms part of the subject matter of International (PCT) Patent Application No. PCT/AU2010/001403 filed on October 22, 2010 in the name of Propanc Pty Ltd. This international PCT application also includes the priority filings of Australian provisional patent application # 2009905147 and # 2010902655, which were filed on October 22, 2009 and June 17, 2010 respectively (as discussed under the section “Intellectual Property”). The PRP-DCM drug product also forms part of the subject matter of International (PCT) Patent Application No. PCT/AU2010/001403. The Authorized Officer indicated in the Written Opinion issued for this international PCT application, that the patent claims covering the PRP and PRP-DCM products were novel over the prior art cited in the International Search Report. Various national phase applications are being filed in countries around the world based on the above priority applications. |
| · | Market opportunity: Growing demand for new cancer treatments as a result of a rapidly aging population and changing environmental factors in western countries. According to the World Health Organization, all cancers (excluding non-melanoma skin cancer) are expected to increase from 8.2 million annual deaths in 2012 to over 10 million annual deaths by 2020, exceeding 13 million annual deaths by 2030. |
Magna Financing
On October 28, 2015 (the “Closing Date”), Propanc Health Group Corporation, a Delaware corporation (the “Company”), entered into a securities purchase agreement dated as of the Closing Date (the “Purchase Agreement”) with Delafield Investments Limited (the “Purchaser”). The Purchase Agreement provides that, upon the terms and subject to the conditions set forth therein, the Purchaser will invest $4,000,000 (“Investment Amount”) in exchange for an 5% Original Issue Discount Senior Secured Convertible Debenture (the “Debenture”) in the principal amount of $4,400,000 (which includes a 10% original issue discount) and warrants to purchase an aggregate of 26,190,476 shares of the Company’s common stock, par value $0.001 per share, for an exercise price of $0.60 per share for a period of four (4) years from the Closing Date (the “Warrant” and such transactions the “October Financing”). In connection with the execution of the Purchase Agreement and the October Financing, the Company and the Purchaser, as applicable also entered into that Debenture, Warrant, Registration Rights Agreement and Security Agreement, each dated as of October 28, 2015, and the Deposit Control Account Agreement, dated November 12, 2015 (all such documents along with the Purchase Agreement, the “October Financing Documents”).
The Company filed a registration statement on Form S-1 on November 23, 2015, which was deemed effective on December 10, 2015, pursuant to which the Company previously registered an aggregate of 98,404,985 shares of the Company's Common Stock consisting of: (i) 72,214,509 shares underlying the Debenture; and (ii) 26,190,476 shares of Common Stock issuable upon exercise of the Warrant (the “Prior Registration Statement”).
Under the terms of the Debenture, the Company was entitled to a reduction in the principal amount of the October 2015 Financing of (i) $25,000 upon the Company’s filing on the Prior Registration Statement within the time period specified and (ii) $25,000 upon the effectiveness of the Prior Registration Statement within the time period specified. Both of these conditions were met resulting in a $50,000 reduction of the principal amount of the October 2015 Financing such that the current aggregate principal amount is $4,350,000 as of the date of this Registration Statement (the “Principal Amount”). Any references to the “principal amount” or the defined term “Principal Amount” used in this Registration Statement shall refer to the reduced Principal Amount as described herein.
2
Addendum to Magna Financing
On March 11, 2016, the Company entered into an Addendum with the Purchaser pursuant to which the Company and the Purchaser agreed to certain new terms with respect to the transactions contemplated by Purchase Agreement (the “Addendum”). The description of the Debenture below reflects the terms of the Debenture as modified by the Addendum.
Debenture
The Debenture has a 10% original issue discount and matures on October 28, 2016. The Principal Amount of the Debenture accrues interest at the rate of 5% per annum, payable quarterly in cash (or if certain conditions are met, in stock at the Company’s option) on January 1, April 1, July 1 and October 1. The Debenture is convertible at any time, in whole or in part, at the Purchaser’s option into shares of the Company’s common stock, par value $0.001 per share (the “Common Stock”), at a conversion price equal to $0.03 (the “Conversion Price”); provided that in the event that the volume weighted average price per share on any trading day is less than such conversion price, the conversion price will be adjusted to a price per share that is equal to a 22.5% discount to the lowest trading price of the common stock in the 10 trading days prior to the date of conversion. At no time will the Purchaser be entitled to convert any portion of the Debenture to the extent that after such conversion, the Purchaser (together with its affiliates) would beneficially own more than 4.99% of the outstanding shares of Common Stock as of such date.
Warrant
The Warrant is exercisable in whole or in part, at an initial exercise price per share of $0.60, subject to adjustment. The exercise price and number of shares of the Company’s common stock issuable under the Warrant are subject to adjustments for stock dividends, splits, combinations, subsequent rights offerings and pro rata distributions. Any adjustment to the exercise price shall similarly cause the number of warrant shares to be adjusted so that the total value of the Warrant may increase. In the event that the shares subject to the Warrant are not included in an effective registration statement, the Warrant may be exercised on a cashless basis.
Registration Rights Agreement
In connection with the execution of the Purchase Agreement, on the Closing Date, the Company and the Purchaser also entered into a registration rights agreement dated as of the Closing Date (the “Registration Rights Agreement”). Pursuant to the Registration Rights Agreement, the Company agreed to file an initial registration statement (“Registration Statement”) with the SEC to register the resale of the Common Stock into which the Debenture may be converted or the Warrant may be exercised. Such Registration Statement was filed on November 23, 2015 and declared effective on December 10, 2015 (the “Prior Registration Statement”). An aggregate of 98,404,985 shares of the Company's Common Stock were registered pursuant to the Prior Registration Statement consisting of: (i) 72,214,509 shares underlying the Debenture; and (ii) 26,190,476 shares of Common Stock issuable upon exercise of the Warrant.
Security Agreement
In connection with the Purchase Agreement, the Company entered into a Security Agreement dated as of even date therewith with the Purchaser whereby the Company agreed to grant to Purchaser an unconditional and continuing, first priority security interest in all of the assets and property of the Company to secure the prompt payment, performance and discharge in full of all of Company’s obligations under the Debenture, Warrant and the other transaction documents until ten days following the such time as the Registration Statement is declared effective by the SEC and the equity conditions set forth in the Debenture are met.
Company History
Propanc Health Group Corporation, formerly Propanc PTY Ltd., is a development stage enterprise and was incorporated in Melbourne, Victoria Australia on October 15, 2007. Based in Melbourne, Victoria Australia, since inception, substantially all of the efforts of our company have been the development of new cancer treatments targeting high risk patients who need a long term therapy which prevents the cancer from returning and spreading. The Company anticipates establishing global markets for its technologies.
3
On November 23, 2010, Propanc Health Group Corporation was incorporated in the state of Delaware. In January 2011, to reorganize the Company, Propanc Health Group Corporation acquired all of the outstanding shares of Propanc PTY Ltd. on a one-for-one basis making it a wholly-owned subsidiary.
We were formed for the specific purpose of having shareholders of Propanc PTY Ltd. directly owning an interest in a U.S. company. On January 29, 2011, we issued 64,700,525 shares of our common stock in exchange for 64,700,525 shares of Propanc PTY Ltd. common stock.
Where You Can Find Us
Our principal executive offices are located at Level 2, 555 Riversdale Road, Camberwell, VIC, 3124, Australia. Our telephone number is +61 (0)3 9882 0780.
Implications of Being an Emerging Growth Company
We qualify as an emerging growth company as that term is used in the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other burdens that are otherwise applicable generally to public companies. These provisions include:
| ● | A requirement to have only two years of audited financial statements and only two years of related MD&A; |
| ● | Exemption from the auditor attestation requirement in the assessment of the emerging growth company’s internal control over financial reporting under Section 404 of the Sarbanes-Oxley Act of 2002; |
| ● | Reduced disclosure about the emerging growth company’s executive compensation arrangements; and |
| ● | No non-binding advisory votes on executive compensation or golden parachute arrangements. |
We have already taken advantage of these reduced reporting burdens in this prospectus, which are also available to us as a smaller reporting company as defined under Rule 12b-2 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
In addition, Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended (the “Securities Act”) for complying with new or revised accounting standards.
We could remain an emerging growth company for up to five years,
or until the earliest of (i) the last day of the first fiscal year in which our annual gross revenues exceed $1 billion, (ii) the
date that we become a “large accelerated filer” as defined in Rule 12b-2 under the Exchange Act, which would occur
if the market value of our common stock that is held by non-affiliates exceeds $700 million as of the last business day of our
most recently completed second fiscal quarter, or (iii) the date on which we have issued more than $1 billion in non-convertible
debt during the preceding three year period.
THE OFFERING
| Securities Offered (1) | 171,000,000 shares of Common Stock, underlying the Debenture. | |
| Common Stock Outstanding Before the Offering (2): | 584,531,788 | |
| Common Stock Outstanding After the Offering (2): | 755,531,788 |
4
| Quotation of Common Stock | Our common stock is listed for quotation on the OTCQB market under the symbol “PPCH” | |
| Terms of the Offering: | The selling shareholders will determine when and how they will sell the Common Stock offered in this prospectus. | |
| Termination of the Offering: | The offering will conclude upon the earliest of: (i) such time as all of the Common Stock has been sold pursuant to the registration statement of which this prospectus forms a part (the “Registration Statement”); or (ii) such time as all of the Common Stock becomes eligible for resale without volume limitations pursuant to Rule 144 under the Securities Act, or any other rule of similar effect. | |
| Use of proceeds: | We are not selling any shares of the Common Stock covered by this prospectus. As such, we will not receive any of the offering proceeds from the registration of the shares of Common Stock covered by this prospectus. We could, however, receive up to $15,714,285.60 in the event the Warrant is exercised for cash (notwithstanding that such warrants have a cashless exercise feature). We will use the proceeds from the exercise of such warrants for general corporate purposes, which may include, among other things, our working capital needs and other general corporate purposes. | |
| Risk Factors: | The Common Stock offered hereby involves a high degree of risk and should not be purchased by investors who cannot afford the loss of their entire investment. See “Risk Factors” beginning on page 6. |
(1) Based on 584,531,788 shares of Common Stock outstanding as of March 18, 2016
(2) Does not include Common Stock underlying any convertible notes, warrant or option, including ones offered in this registration statement.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
The information contained in this prospectus, including in the documents incorporated by reference into this prospectus, includes some statements that are not purely historical and that are “forward-looking statements.” Such forward-looking statements include, but are not limited to, statements regarding our Company and management’s expectations, hopes, beliefs, intentions or strategies regarding the future, including our financial condition, results of operations, and the expected impact of the offering on the parties’ individual and combined financial performance. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “anticipates,” “believes,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “might,” “plans,” “possible,” “potential,” “predicts,” “projects,” “seeks,” “should,” “will,” “would” and similar expressions, or the negatives of such terms, may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking.
The forward-looking statements contained in this prospectus are based on current expectations and beliefs concerning future developments and the potential effects on the parties and the transaction. There can be no assurance that future developments actually affecting us will be those anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond the parties’ control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements.
5
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below together with the information contained in this prospectus, including our financial statements and the related notes appearing at the end of this prospectus. If any of the following occur, our business, financial condition, results of operations and future growth prospects could be materially adversely affected. In these circumstances, the price of our common stock could decline, and you may lose all of your investment.
RISKS RELATED TO OUR FINANCIAL CONDITION AND OUR NEED FOR ADDITIONAL CAPITAL
Our independent registered accounting firm has expressed concerns about our ability to continue as a going concern. Our ability to continue as a going concern is in substantial doubt absent obtaining adequate new debt or equity financings.
The report of our independent registered accounting firm expresses concern about our ability to continue as a going concern based on the absence of significant revenues, recurring losses from operations and our need for additional financing to fund all of our operations. Working capital limitations continue to impinge on our day-to-day operations, thus contributing to continued operating losses. For the three and six months ended December 31, 2015, we had net losses of $3,562,067 and $4,400,961, respectively. For the years ended June 30, 2015 and 2014, we had net losses of $3,412,754 and $829,564, respectively. Further, as of December 31, 2015, we had $1,168,118 in cash, $0 in receivable accounts and had an accumulated deficit of $25,366,632. As of June 30, 2015, we had $107,627 in cash, $0 in receivable accounts and had an accumulated deficit of $20,965,671.
Based upon our current business plans, we will need considerable cash investments to be successful. Although we recently raised a significant debt financing of approximately $4,000,000 which should give us enough available cash to meet our obligations over the next 12 months, our capital requirements and cash needs are significant and continuing. We can provide no assurance that we will be able to generate a sufficient amount of revenue, if any, from our business in order to achieve profitability. It is not possible at this time for us to predict with assurance the potential success of our business. The revenue and income potential of our proposed business and operations are unknown. If we cannot continue as a viable entity, we may be unable to continue our operations and you may lose some or all of your investment in our common stock.
We have incurred significant losses since our inception. We expect to incur losses for the foreseeable future and never achieve or maintain profitability.
Since inception, we have incurred significant operating losses. Our net loss was $3,562,067 and $4,400,961, respectively for the three and six months ended December 31, 2015, and was $3,412,754 for the year ended June 30, 2015. As of December 31, 2015 and June 30, 2015, we had a deficit accumulated during the development phase of $25,366,632 and $20,965,671, respectively. To date, we have not generated any revenues and have financed our operations with funds obtained from private financings and related party transactions with directors and other officers. From October 2007, we have devoted substantially all of our efforts to research and development of our product candidates and from June 20, 2015 to November 12, 2015, we did a number of laboratory studies examining the anti-cancer effects of our lead product candidates, which has resulted in additional patent specifications prepared for filing and implementing plans to progress our lead product candidate into human studies. More recently, from January to February 2016, we completed a dose range finding study in rodents and will be conducting a scientific advice meeting with the UK regulatory agency to discuss formal GLP (Good Laboratory Practice) animal safety/toxicology studies and Phase I and II clinical trials for our lead product. We expect that it will be many years, if ever, before we have a product candidate ready for commercialization. We expect to incur significant expenses and increasing operating losses for the foreseeable future. We anticipate that our expenses will increase substantially if and as we:
| · | Continue to develop and progress our lead product candidate into human trials; |
| · | continue our research and development efforts; |
| · | initiate clinical trials for our product candidates; |
| · | seek regulatory approvals for our product candidates that successfully complete clinical trials; |
| · | establish a sales, marketing and distribution infrastructure; |
| · | maintain, expand and protect our intellectual property portfolio; and |
| · | add operational, financial and management information systems and personnel, including personnel to support our product development and planned future commercialization efforts. |
6
To become profitable, we must develop and eventually commercialize a product or products with significant market potential. The will require us to successfully complete pre-clinical testing and clinical trials of our product candidates, obtain market approval for our product candidates and manufacturing, marketing and selling those products that we obtain market approval for. We might not succeed in any one or a number of these activities, and even if we do, may never generate revenues that are significant or large enough to achieve profitability. Our failure to become and remain profitable would decrease our value and could impair our ability to raise capital, maintain our research and development efforts, expand our business or continue our operations. A decline in the value of our company could also cause you to lose all or part of your investment.
Our short operating history may make it difficult for you to evaluate the success of our business to date and to assess our future viability.
We are an early stage company. We commenced active operations in the second half 2010. Our operations to date have been limited to establishing our research programs and partnerships, building our intellectual property portfolio and deepening our scientific understanding of our product candidates. We have not yet demonstrated our ability to successfully complete any clinical trials, including large-scale, pivotal clinical trials, obtain marketing approvals, manufacture a commercial scale product, or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. It will take a number of years for our product to be made available for the treatment of cancer. Given our short operating history compared to the timeline required to fully develop a new drug, you are cautioned about making any predictions on our future success or viability based on our activities or results to date. In addition, as a new business, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors. We will need to transition from a company with a research focus to a company capable of supporting commercial activities. We may not be successful in such a transition.
We will continue to need substantial additional funding. If we are unable to raise capital when needed, we would be forced to delay, reduce or eliminate our product development programs or commercialization efforts.
We expect our expenses to increase in connection with our ongoing activities, particularly as we expand our research and development activities and initiate clinical trials of, and seek marketing approval for, our product candidates. In addition, if we obtain marketing approval for any of our product candidates, we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing and distribution. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we would be forced to delay, reduce or eliminate our research and development programs or any future commercialization efforts.
Our future capital requirements will depend on many factors, including:
| · | assuming favorable clinical results, the cost, timing and outcome of our efforts to seek approval in the United States and elsewhere in the world, including to fund the preparation and filing of regulatory submissions with the Food and Drug Administration (“FDA”) and other regulatory agencies worldwide; |
| · | the scope, progress and, results of our other ongoing and potential future clinical trials; |
| · | the extent to which we acquire or in-license other products and technologies; |
| · | the costs, timing and outcome of regulatory review of our product candidates; |
| · | the costs of future commercialization activities, including product sales, marketing, manufacturing and distribution, for any of our product candidates for which we receive marketing approval; |
| · | revenue, if any, received from commercial sales of our product candidates, should any of our product candidates receive marketing approval; |
| · | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims; and |
| · | our ability to establish collaborations on favorable terms, if at all. |
Identifying potential product candidates and conducting preclinical testing and clinical trials is a time-consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain marketing approval and achieve product sales. In addition, our product candidates, if approved, may not achieve commercial success. Our commercial revenues, if any, will be derived from sales of products that we do not expect to be commercially available for many years, if at all. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Adequate additional financing may not be available to us on acceptable terms, or at all.
7
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, collaborations, strategic alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or debt securities, including convertible debt securities the ownership interest of our existing stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our existing stockholders. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
If we raise additional funds through collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Risks related to the discovery, development and commercialization of our product candidates
Because our product candidates are in the early stages of development and may never lead to commercially viable drugs, you may lose your investment.
We are a research and development company presently focused on the development of new cancer treatments, all of which are at an early stage of development, which may be effective in treating cancer and have use in reducing the risk of cancer recurrence. Our drug development methods may not lead to commercially viable drugs for any of several reasons. For example, we may fail to identify appropriate compounds, our drug candidates may fail to be safe and effective in additional preclinical or clinical trials, or we may have inadequate financial or other resources to pursue discovery and development efforts for new drug candidates. Our product candidates will require significant additional development, clinical trials, regulatory clearances and additional investment by us before they can be commercialized. If, for any of these reasons, we are unsuccessful at commercializing our drug candidates, you may lose your investment.
At present, both our lead product candidates, PRP and PRP-DCM are still in preclinical development. A formal GLP toxicology study will be completed to ensure the safety of our product candidate, PRP, prior to entering into clinical trials for testing in humans. Further work is also needed for PRP-DCM to better understand which animal models are most appropriate, and determining the optimal combination for PRP-DCM, prior to proceeding into formal preclinical studies and into clinical trials.
Our products may cause undesirable side effects that could limit their use, require their removal from the market or prevent further development.
Side effects that may be caused by our products could interrupt, delay or halt our development programs, including clinical trials, and could result in adverse regulatory action by the FDA or other regulatory authorities. More severe side effects associated with our products may be also observed in the future. Even if we are able to complete the development of a new product and obtain any required regulatory approval, undesirable side effects could prevent us from achieving or maintaining market acceptance of the product or could substantially increase the costs and expenses of commercializing the product. Negative publicity concerning our products, whether accurate or inaccurate, could also reduce market or regulatory acceptance of our products, which could result in decreased product demand, removal from the market or an increased number of product liability claims, whether or not such claims have merit.
8
Because successful development of our products is uncertain, our results of operations may be materially harmed.
Our development of current and future product candidates is subject to the risks of failure and delay inherent in the development of new pharmaceutical products and products based on new technologies, including but not limited to the following:
| · | delays in product development, clinical testing, or manufacturing; |
| · | unplanned expenditures in product development, clinical testing or manufacturing; |
| · | unexpected scientific, non-clinical or clinical findings relating to safety or efficacy; |
| · | failure to receive regulatory approvals; |
| · | emergence of superior or equivalent products; |
| · | inability to manufacture our product candidates on a commercial scale on our own, or in collaboration with third parties; and |
| · | failure to achieve market acceptance. |
Because of these risks, our development efforts may not result in any commercially viable products. If a significant portion of these development efforts are not successfully completed, required regulatory approvals will not be obtained, or if any approved products are not commercialized successfully, our business, financial condition, and results of operations may be materially harmed.
Additional preclinical testing and clinical trials of our product candidates may not be successful if we are unable to commercialize our product candidates or experience significant delays in doing so, our business may be harmed.
We have conducted a variety of pre-clinical studies, which have provided evidence supporting the potential therapeutic utility of our lead product candidates, PRP and PRP-DCM. Studies include the in vitro assessment of these product’s key components on cell growth and differentiation, and in vitro combination assays identifying synergistic effects by optimizing the ratios between the key components. In addition, we, together with our scientific founder, Dr. Julian Kenyon, have undertaken a retrospective analysis of cancer patients treated with PRP under UK and Australian compassionate access schemes. This review has generated clinical evidence supportive of the further development of PRP as a potential therapeutic for cancer.
However, before regulatory approval can be obtained for the commercial sale of PRP, or the other product candidates currently under development by us, we will be required to complete formal preclinical studies and then comprehensive clinical trials in order to demonstrate the product’s safety, tolerability and efficacy. Regulatory approval to market a new product will only be obtained once we can demonstrate to the satisfaction of the applicable regulatory authority that the product candidate has an acceptable safety profile, is effective in treating the target indication and otherwise meets the appropriate standards required by regulators for approval.
Clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. A failure of one or more clinical trials can occur at any stage of testing. The outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their products.
We may experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent our ability to receive marketing approval or commercialize our product candidates, including:
| · | regulators or institutional review boards may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site; |
| · | we may have delays in reaching or fail to reach agreement on acceptable clinical trial contracts clinical trial protocols with prospective trial sites; |
| · | clinical trials of our product candidates may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical trials or abandon product development programs; |
9
| · | the number of patients required for clinical trials of our product candidates may be larger than we anticipate, enrollment in these clinical trials may be slower than we anticipate or participants may drop out of these clinical trials at a higher rate than we anticipate; |
| · | our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all; | |
| · | we might have to suspend or terminate clinical trials of our product candidates for various reasons, including a finding that the participants are being exposed to unacceptable health risks; |
| · | regulators or institutional review boards may require that we or our investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks; |
| · | the cost of clinical trials of our product candidates may be greater than we anticipate; |
| · | the supply or quality of our product candidates or other materials necessary to conduct clinical trials of our product candidates may be insufficient or inadequate; and |
| · | our product candidates may have undesirable side effects or other unexpected characteristics, causing us or our investigators, regulators or institutional review boards to suspend or terminate the trials. |
If we are required to conduct additional clinical trials or other testing of our product candidates beyond those that we currently contemplate, if we are unable to successfully complete clinical trials of our product candidates or other testing, if the results of these trials or tests are not positive or are only modestly positive or if there are safety concerns, we may:
| · | be delayed in obtaining marketing approval for our product candidates; |
| · | not obtain marketing approval at all; |
| · | not obtain marketing approval at all; |
| · | obtain approval for indications or patient populations that are not as broad as intended or desired; |
| · | obtain approval with labeling that includes significant use or distribution restrictions or safety warnings, including boxed warnings; |
| · | be subject to additional post-marketing testing requirements; or |
| · | have the product removed from the market after obtaining marketing approval. |
Any delay in, or termination of, our clinical trials may result in increased development costs for our products, which would cause the market price of our shares to decline and limit our ability to obtain additional financing and, ultimately, our ability to commercialize our products and generate product revenues. Any change in, or termination of, our clinical trials could materially harm our business, financial condition and results of operations.
If we experience delays or difficulties in the enrollment of patients in clinical trials, our receipt of necessary regulatory approvals could be delayed or prevented.
We may not be able to initiate or continue clinical trials for our product candidates if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the U.S. Food and Drug Administration, or FDA, or similar regulatory authorities outside the United States. In addition, there are a number of ongoing clinical trials for product candidates treating cancer. Patients who would otherwise be eligible for our clinical trials may instead enroll in clinical trials of our competitors' product candidates, particularly if they view such treatments to be more conventional and established.
Patient enrollment is affected by other factors including:
| · | severity of the disease under investigation; |
| · | eligibility criteria for the study in question; |
| · | perceived risks and benefits of the product candidate under study; |
| · | efforts to facilitate timely enrollment in clinical trials; |
| · | patient referral practices of physicians; |
| · | the ability to monitor patients adequately during and after treatment; and | |
| · | proximity and availability of clinical trial sites for prospective patients. |
10
Our inability to enroll a sufficient number of patients for our clinical trials would result in significant delays or may require us to abandon one or more clinical trials altogether. Enrollment delays in our clinical trials may result in increased development costs for our product candidates, which would cause the value of our company to decline and limit our ability to obtain additional financing.
If serious adverse or unexpected side effects are identified during the development of our product candidates, we may need to abandon or limit our development of some of our product candidates.
All of our product candidates are in preclinical development or early stages of clinical development and their risk of failure is high. It is impossible to predict when or if any of our product candidates will prove effective or safe in humans or will receive marketing approval. If our product candidates are associated with undesirable side effects or have characteristics that are unexpected, we may need to abandon their development or limit development to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective. Many compounds that initially showed promise in early stage testing for treating cancer have later been found to cause side effects that prevented further development of the compound.
If we fail to obtain regulatory approval in jurisdictions outside the United States, we will not be able to market our products in those jurisdictions.
We intend to seek regulatory approval for our product candidates in a number of countries outside of the United States and expect that these countries will be important markets for our products, if approved. Marketing our products in these countries will require separate regulatory approvals in each market and compliance with numerous and varying regulatory requirements. The regulations that apply to the conduct of clinical trials and approval procedures vary from country to country and may require additional testing. Moreover, the time required to obtain approval may differ from that required to obtain FDA approval. In addition, in many countries outside the United States, drugs must be approved for reimbursement before it can be approved for sale in that country. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval. We may not obtain foreign regulatory approvals on a timely basis, if at all. We may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our products in any foreign market.
Even if regulatory approval is obtained, our products will be subject to extensive post-approval regulation.
Once a product is approved, numerous post-approval requirements apply, including but not limited to requirements relating to manufacturing, labeling, packaging, advertising and record keeping. Even if regulatory approval of a product is obtained, the approval may be subject to limitations on the uses for which the product may be marketed, or contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the product. Any such post-approval requirement could reduce our revenues, increase our expenses and render the approved product candidate not commercially viable. In addition, as clinical experience with a drug expands after approval because it is typically used by a greater number and more diverse group of patients after approval than during clinical trials, side effects and other problems may be observed after approval that were not seen or anticipated during pre-approval clinical trials or other studies. Any adverse effects observed after the approval and marketing of a product candidate could result in limitations on the use of such approved product or its withdrawal from the marketplace. Absence of long-term safety data may also limit the approved uses of our products. If we fail to comply with the regulatory requirements of the applicable regulatory authorities, or if previously unknown problems with any approved commercial products, manufacturers or manufacturing processes are discovered, we could be subject to administrative or judicially imposed sanctions or other setbacks, including:
| ● | restrictions on the products, manufacturers or manufacturing processes; |
| ● | warning letters and untitled letters; |
| ● | civil penalties and criminal prosecutions and penalties; |
| ● | fines; |
| ● | injunctions; |
11
| ● | product seizures or detentions; |
| ● | import or export bans or restrictions; |
| ● | voluntary or mandatory product recalls and related publicity requirements; |
| ● | suspension or withdrawal of regulatory approvals; |
| ● | total or partial suspension of production; and |
| ● | refusal to approve pending applications for marketing approval of new products or of supplements to approved applications. |
If we are slow or unable to adapt to changes in existing regulatory requirements or the promulgation of new regulatory requirements or policies, we or our licensees may lose marketing approval for our products which will impact our ability to conduct business in the future.
Even if any of our product candidates receive marketing approval, they may fail to achieve the degree of market acceptance by physicians, patients, healthcare payors and others in the medical community necessary for commercial success.
If any of our product candidates receive marketing approval, they may nonetheless fail to gain sufficient market acceptance by physicians, patients, healthcare payors and others in the medical community. For example, current cancer treatments like chemotherapy and radiation therapy are well established in the medical community, and doctors may continue to rely on these treatments. If our product candidates do not achieve an adequate level of acceptance, we may not generate significant product revenues and we may not become profitable. The degree of market acceptance of our product candidates, if approved for commercial sale, will depend on a number of factors, including:
| · | efficacy and potential advantages compared to alternative treatments; |
| · | the ability to offer our products for sale at competitive prices; |
| · | convenience and ease of administration compared to alternative treatments; |
| · | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
| · | the strength of marketing and distribution support; |
| · | sufficient third-party coverage or reimbursement; and |
| · | the prevalence and severity of any side effects. |
We intend to conduct business in multiple countries, where we will be exposed to political, economic and other risks that may adversely affect our business.
Currently our headquarters are in Australia, but we intend to penetrate other markets in the future. At such time, we will therefore be exposed to risks inherent in international operations. These risks include, but are not limited to:
| · | changes in general economic, social and political conditions; |
| · | adverse tax consequences; | |
| · | the difficulty of enforcing agreements and collecting receivables through certain legal systems; |
| · | inadequate protection of intellectual property; |
| · | required compliance with a variety of laws and regulations in jurisdictions outside of Australia, including labor and tax laws; and |
| · | customers outside of the United States may have longer payment cycles. |
Our business success depends in part on our ability to anticipate and effectively manage these and other regulatory, economic, social and political risks inherent in a multinational business. We cannot assure you that we will be able to effectively manage these risks or that they will not have a material adverse effect on our multinational business or on our business as a whole.
12
If, in the future, we are unable to establish sales and marketing capabilities or enter into agreements with third parties to sell and market our product candidates, we may not be successful in commercializing our product candidates if and when they are approved.
We do not have a sales or marketing infrastructure and have no experience in the sale, marketing or distribution of pharmaceutical products. To achieve commercial success for any approved product, we must either develop a sales and marketing organization or outsource these functions to third parties. In the future, we may choose to build a focused sales and marketing infrastructure to market or co-promote some of our product candidates if and when they are approved.
There are risks involved with both establishing our own sales and marketing capabilities and entering into arrangements with third parties to perform these services. For example, recruiting and training a sales force is expensive and time consuming and could delay any product launch. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
Factors that may inhibit our efforts to commercialize our products on our own include:
| · | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
| · | the inability of sales personnel to obtain access to physicians or persuade adequate numbers of physicians to prescribe any future products; |
| · | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
| · | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
If we enter into arrangements with third parties to perform sales, marketing and distribution services, our product revenues or the profitability of these product revenues to us are likely to be lower than if we were to market and sell any products that we develop ourselves. In addition, we may not be successful in entering into arrangements with third parties to sell and market our product candidates or may be unable to do so on terms that are favorable to us. We likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively. If we do not establish sales and marketing capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of new drug products is highly competitive. We face competition with respect to our current product candidates, and will face competition with respect to any product candidates that we may seek to develop or commercialize in the future, from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. There are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of products for the treatment of the disease indications for which we are developing our product candidates. Some of these competitive products and therapies are based on scientific approaches that are the same as or similar to our approach, and others are based on entirely different approaches. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
We are developing our product candidates for the treatment of cancer. There are a variety of available therapies marketed for cancer. In many cases, these drugs are administered in combination to enhance efficacy. Some of these drugs are branded and subject to patent protection, and others are available on a generic basis. Many of these approved drugs are well-established therapies and are widely accepted by physicians, patients and third-party payors. Insurers and other third-party payors may also encourage the use of generic products. We expect that if our product candidates are approved, they will be priced at a significant premium over competitive generic products. This may make it difficult for us to achieve our business strategy of using our product candidates in combination with existing therapies or replacing existing therapies with our product candidates.
13
There are also a number of products in clinical development by third parties to treat and prevent metastatic cancer. These companies include divisions of large pharmaceutical companies, including Astellas Pharma US, Inc., Sanofi-Aventis US LLC, GlaxoSmithKline plc, Boehringer Ingelheim GmbH, Pfizer Inc. and others. There are also biotechnology companies of various sizes that are developing therapies against cancer stem cells, or CSCs (i.e. cancer cells which have transformed to become motile and invasive, triggering metastasis, and are chemo-resistant), including Verastem, OncoMed Pharmaceuticals, Inc., Boston Biomedical, Inc. and Stemline Therapeutics, Inc. Our competitors may develop products that are more effective, safer, more convenient or less costly than any that we are developing or that would render our product candidates obsolete or non-competitive. In addition, our competitors may discover biomarkers that more efficiently measure their effectiveness to treat and prevent metastatic cancer, which may give them a competitive advantage in developing potential products. Our competitors may also obtain marketing approval from the FDA or other regulatory authorities for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market.
Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. In addition, to the extent that product or product candidates of our competitors demonstrate serious adverse side effects or are determined to be ineffective in clinical trials, the development of our product candidates could be negatively impacted.
Even if we are able to commercialize any product candidates, the products may become subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, which would harm our business.
The regulations that govern marketing approvals, pricing and reimbursement for new drug products vary widely from country to country. In the United States, recently passed legislation may significantly change the approval requirements in ways that could involve additional costs and cause delays in obtaining approvals. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we might obtain marketing approval for a product in a particular country, but then be subject to price regulations that delay our commercial launch of the product, possibly for lengthy time periods, and negatively impact the revenues we are able to generate from the sale of the product in that country. Adverse pricing limitations may hinder our ability to recoup our investment in one or more product candidates, even if our product candidates obtain marketing approval.
Our ability to commercialize any products successfully also will depend in part on the extent to which reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers and other organizations. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. We cannot be sure that reimbursement will be available for any product that we commercialize and, if reimbursement is available, the level of reimbursement. Reimbursement may impact the demand for, or the price of, any product candidate for which we obtain marketing approval. Obtaining reimbursement for our products may be particularly difficult because of the higher prices often associated with drugs administered under the supervision of a physician. If reimbursement is not available or is available only to limited levels, we may not be able to successfully commercialize any product candidate for which we obtain marketing approval.
14
There may be significant delays in obtaining reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or similar regulatory authorities outside the United States. Moreover, eligibility for reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. Our inability to promptly obtain coverage and profitable payment rates from both government-funded and private payors for any approved products that we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of any products that we may develop.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials and will face an even greater risk if we commercially sell any products that we may develop. If we cannot successfully defend ourselves against claims that our product candidates or products caused injuries, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| · | decreased demand for any product candidates or products that we may develop; |
| · | injury to our reputation and significant negative media attention; | |
| · | withdrawal of clinical trial participants; |
| · | significant costs to defend the related litigation; |
| · | substantial monetary awards to trial participants or patients; |
| · | loss of revenue; and |
| · | the inability to commercialize any products that we may develop. |
We currently do not hold product liability insurance coverage. We shall take out product liability insurance prior to our first clinical trial in the aggregate, with a per incident limit of $5 million, which may not be adequate to cover all liabilities that we may incur. We may need to increase our insurance coverage as we initiate additional clinical trials or upon the commercialization of our product candidates, if ever. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
RISKS RELATED TO OUR DEPENDENCE ON THIRD PARTIES
We may depend on collaborations with third parties for the development and commercialization of our product candidates. If those collaborations are not successful, we may not be able to capitalize on the market potential of these product candidates.
We may seek third-party collaborators for the development and commercialization of our product candidates. Our likely collaborators for any collaboration arrangements include large and mid-size pharmaceutical companies, regional and national pharmaceutical companies and biotechnology companies. If we do enter into any such arrangements with any third parties, we will likely have limited control over the amount and timing of resources that our collaborators dedicate to the development or commercialization of our product candidates. Our ability to generate revenues from these arrangements will depend on our collaborators' abilities to successfully perform the functions assigned to them in these arrangements.
15
Collaborations involving our product candidates would pose the following risks to us:
| · | collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations; |
| · | collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in the collaborator's strategic focus or available funding or external factors such as an acquisition that diverts resources or creates competing priorities; |
| · | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; |
| · | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours; |
| · | a collaborator with marketing and distribution rights to one or more products may not commit sufficient resources to the marketing and distribution of such product or products; | |
| · | collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our proprietary information or expose us to potential litigation; |
| · | disputes may arise between the collaborators and us that result in the delay or termination of the research, development or commercialization of our products or product candidates or that result in costly litigation or arbitration that diverts management attention and resources; and |
| · | collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates. |
Collaboration agreements may not lead to development or commercialization of product candidates in the most efficient manner or at all. If a present or future collaborator of ours were to be involved in a business combination, the continued pursuit and emphasis on our product development or commercialization program could be delayed, diminished or terminated.
If we are not able to establish collaborations, we may have to alter our development and commercialization plans.
Our drug development programs and the potential commercialization of our product candidates will require substantial additional cash to fund expenses. For some of our product candidates, we may decide to collaborate with pharmaceutical and biotechnology companies for the development and potential commercialization of those product candidates.
We face significant competition in seeking appropriate collaborators. Whether we reach a definitive agreement for collaboration will depend, among other things, upon our assessment of the collaborator's resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator's evaluation of a number of factors. Those factors may include the design or results of clinical trials, the likelihood of approval by the FDA or similar regulatory authorities outside the United States, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on and whether such collaboration could be more attractive than the one with us for our product candidate. We may also be restricted under existing license agreements from entering into agreements on certain terms with potential collaborators. Collaborations are complex and time-consuming to negotiate and document. In addition, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators.
16
We may not be able to negotiate collaborations on a timely basis, on acceptable terms, or at all. If we are unable to do so, we may have to curtail the development of such product candidate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms or at all. If we do not have sufficient funds, we may not be able to further develop our product candidates or bring them to market and generate product revenue.
We contract with third parties for the manufacture of our product candidates and for compound formulation research and these third parties may not perform satisfactorily.
We do not have any manufacturing facilities or personnel. We currently obtain all of our supply of our product candidates for clinical development from third-party manufacturers or third-party collaborators, and we expect to continue to rely on third parties for the manufacture of clinical and, if necessary, commercial quantities of our product candidates. In addition, we currently rely on third parties for the development of various formulations of our product candidates. We obtain our supplies from these manufacturers on a purchase order basis, and we do not have any long term supply agreements in place. This reliance on third parties increases the risk that we will not have sufficient quantities of our product candidates or such quantities at an acceptable cost or quality, which could delay, prevent or impair our development or commercialization efforts.
Any of these third parties may terminate their engagement with us at any time. We do not currently have arrangements in place for redundant supply or a second source for bulk drug substance. Even if we are able to establish agreements with third-party manufacturers, reliance on third-party manufacturers entails additional risks, including:
| · | collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations; |
| · | reliance on the third party for regulatory compliance and quality assurance; |
| · | the possible breach of the manufacturing agreement by the third party, including the misappropriation of our proprietary information, trade secrets and know-how; |
| · | the possible termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us; and |
| · | disruptions to the operations of our manufacturers or suppliers caused by conditions unrelated to our business or operations, including the bankruptcy of the manufacturer or supplier or a catastrophic event affecting our manufacturers or suppliers. |
Third-party manufacturers may not be able to comply with current good manufacturing practices, or cGMP, regulations or similar regulatory requirements outside the United States. Our failure, or the failure of our third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of product candidates or products, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of our products and harm our business and results of operations.
Any products that we may develop may compete with other product candidates and products for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that might be capable of manufacturing for us. If our current contract manufacturers cannot perform as agreed, we may be required to replace that manufacturer. Although we believe that there are several potential alternative manufacturers who could manufacture our product candidates, we may incur added costs and delays in identifying and qualifying any such replacement, as well as producing the drug product. In addition, we have to enter into technical transfer agreements and share our know-how with the third-party manufacturers, which can be time-consuming and may result in delays.
17
Our current and anticipated future dependence upon others for the manufacture of our product candidates or products may adversely affect our future profit margins and our ability to commercialize any products that receive marketing approval on a timely and competitive basis.
RISKS RELATED TO OUR INTELLECTUAL PROPERTY
If we fail to comply with our obligations under our intellectual property licenses with third parties, we could lose license rights that are important to our business.
We are a party to a joint commercialization agreement with the University of Bath, and expect to enter into license agreements in the future. If we fail to comply with our obligations this commercialization agreement and any future license we may enter into in the future, such licensors may have the right to terminate these agreements, in which event we might not be able to market any product that is covered by the agreements, or to convert the exclusive licenses to non-exclusive licenses, which could materially adversely affect the value of the product candidate being developed under these license agreements. Termination of license agreements or reduction or elimination of our licensed rights may result in our having to negotiate new or reinstated licenses with less favorable terms. If the University of Bath were to terminate the agreement with us for any reason, we would lose the rights, title and interest for commercializing PRP and/or PRP-DCM as a treatment for cancer, or related fields.
If we are unable to obtain and maintain patent protection for our technology and products, or if our licensors are unable to obtain and maintain patent protection for the technology or products that we license from them, or if the scope of the patent protection obtained is not sufficiently broad, our competitors could develop and commercialize technology and products similar or identical to ours, and our ability to successfully commercialize our technology and products may be adversely affected.
Our success depends in large part on our and our licensors' ability to obtain and maintain patent protection in the United States and other countries with respect to our proprietary technology and products. We seek to protect our proprietary position by filing patent applications in the United States and abroad related to our novel technologies and products that are important to our business. We cannot be certain that any patents will issue with claims that cover our proprietary technology or product candidates.
The patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. Moreover, in some circumstances, we do not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the patents, covering technology or products that we license from third parties and are reliant on our licensors. If such licensors fail to maintain such patents, or lose rights to those patents, the rights we have licensed may be reduced or eliminated.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our and our licensors' patent rights are highly uncertain. Our and our licensors' pending and future patent applications may not result in patents being issued which protect our technology or products or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection.
The laws of foreign countries may not protect our rights to the same extent as the laws of the United States. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore we cannot be certain that we or our licensors were the first to make the inventions claimed in our owned or licensed patents or pending patent applications, or that we or our licensors were the first to file for patent protection of such inventions.
18
Assuming the other requirements for patentability are met, in the United States, for patents that have an effective filing date prior to March 15, 2013, the first to make the claimed invention is entitled to the patent, while outside the United States, the first to file a patent application is entitled to the patent. In March 2013, the United States transitioned to a first inventor to file system in which, assuming the other requirements for patentability are met, the first inventor to file a patent application will be entitled to the patent. We may be subject to a third party preissuance submission of prior art to the U.S. Patent and Trademark Office, or become involved in opposition, derivation, reexamination, inter parties review or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights.
Even if our owned and licensed patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
We may become involved in lawsuits to protect or enforce our patents, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our patents. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours is invalid or unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, our licensors may have rights to file and prosecute such claims and we are reliant on them.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.
Our commercial success depends upon our ability and the ability of our collaborators to develop, manufacture, market and sell our product candidates and use our proprietary technologies without infringing the proprietary rights of third parties. We have yet to conduct comprehensive freedom-to-operate searches to determine whether our use of certain of the patent rights owned by or licensed to us would infringe patents issued to third parties. We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology, including interference proceedings before the U.S. Patent and Trademark Office. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future. If we are found to infringe a third party's intellectual property rights, we could be required to obtain a license from such third party to continue developing and marketing our products and technology. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, we could be found liable for monetary damages. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
19
Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for some of our technology and products, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
RISKS RELATED TO REGULATORY APPROVAL OF OUR PRODUCT CANDIDATES AND OTHER LEGAL COMPLIANCE MATTERS
If we are not able to obtain, or if there are delays in obtaining, required regulatory approvals, we will not be able to commercialize our product candidates, and our ability to generate revenue will be materially impaired.
Our product candidates and the activities associated with their development and commercialization, including their design, testing, manufacture, safety, efficacy, recordkeeping, labeling, storage, approval, advertising, promotion, sale and distribution, are subject to comprehensive regulation by the FDA and other regulatory agencies in the United States and by comparable authorities in other countries. Failure to obtain marketing approval for a product candidate will prevent us from commercializing the product candidate. We have not received approval to market any of our product candidates from regulatory authorities in any jurisdiction. We have only limited experience in filing and supporting the applications necessary to gain marketing approvals and expect to rely on third-party contract research organizations to assist us in this process. Securing FDA approval requires the submission of extensive preclinical and clinical data and supporting information to the FDA for each therapeutic indication to establish the product candidate's safety and efficacy. Securing FDA approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the FDA. Our product candidates may not be effective, may be only moderately effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude our obtaining marketing approval or prevent or limit commercial use.
20
The process of obtaining marketing approvals, both in the United States and abroad, is expensive, may take many years if additional clinical trials are required, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved. Changes in marketing approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application, may cause delays in the approval or rejection of an application. The FDA has substantial discretion in the approval process and may refuse to accept any application or may decide that our data is insufficient for approval and require additional preclinical, clinical or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent marketing approval of a product candidate. Any marketing approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
If we experience delays in obtaining approval or if we fail to obtain approval of our product candidates, the commercial prospects for our product candidates may be harmed and our ability to generate revenues will be materially impaired.
Failure to obtain marketing approval in international jurisdictions would prevent our product candidates from being marketed abroad.
We intend to seek regulatory approval for our product candidates in a number of countries outside of the United States and expect that these countries will be important markets for our products, if approved. In order to market and sell our products in the European Union and many other jurisdictions, we or our third-party collaborators must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain FDA approval. The regulatory approval process outside the United States generally includes all of the risks associated with obtaining FDA approval. In addition, in many countries outside the United States, it is required that the product be approved for reimbursement before the product can be approved for sale in that country. We or these third parties may not obtain approvals from regulatory authorities outside the United States on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the United States does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our products in any market.
Any product candidate for which we obtain marketing approval could be subject to restrictions or withdrawal from the market and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our products, when and if any of them are approved.
Any product candidate for which we obtain marketing approval, along with the manufacturing processes, post-approval clinical data, labeling, advertising and promotional activities for such product, will be subject to continual requirements of and review by the FDA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, registration and listing requirements, cGMP requirements relating to quality control, quality assurance and corresponding maintenance of records and documents, requirements regarding the distribution of samples to physicians and recordkeeping. Even if marketing approval of a product candidate is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the product. The FDA closely regulates the post-approval marketing and promotion of drugs to ensure drugs are marketed only for the approved indications and in accordance with the provisions of the approved labeling. The FDA imposes stringent restrictions on manufacturers' communications regarding off-label use and if we do not market our products for their approved indications, we may be subject to enforcement action for off-label marketing.
In addition, later discovery of previously unknown problems with our products, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various results, including:
| · | restrictions on such products, manufacturers or manufacturing processes; |
21
| · | restrictions on the labeling or marketing of a product; |
| · | restrictions on product distribution or use; |
| · | requirements to conduct post-marketing clinical trials; |
| · | warning or untitled letters; |
| · | withdrawal of the products from the market; |
| · | refusal to approve pending applications or supplements to approved applications that we submit; |
| · | recall of products; |
| · | fines, restitution or disgorgement of profits or revenue; |
| · | suspension or withdrawal of marketing approvals; |
| · | refusal to permit the import or export of our products; |
| · | product seizure; or |
| · | injunctions or the imposition of civil or criminal penalties. |
Our relationships with customers and third-party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and third-party payors play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our future arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations, include the following:
| · | the federal healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid; |
| · | the federal False Claims Act imposes criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; | |
| · | the federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| · | the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; |
| · | the federal transparency requirements under the Health Care Reform Law requires manufacturers of drugs, devices, biologics and medical supplies to report to the Department of Health and Human Services information related to physician payments and other transfers of value and physician ownership and investment interests; and |
| · | analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures. |
22
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other providers or entities with whom we expect to do business are found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Recently enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain marketing approval.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or Medicare Modernization Act, changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for physician-administered drugs. In addition, this legislation provided authority for limiting the number of drugs that will be covered in any therapeutic class. Cost reduction initiatives and other provisions of this legislation could decrease the coverage and price that we receive for any approved products. While the Medicare Modernization Act applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the Medicare Modernization Act may result in a similar reduction in payments from private payors.
More recently, in March 2010, President Obama signed into law the Health Care Reform Law, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for health care and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. Effective October 1, 2010, the Health Care Reform Law revises the definition of "average manufacturer price" for reporting purposes, which could increase the amount of Medicaid drug rebates to states. Further, the new law imposes a significant annual fee on companies that manufacture or import branded prescription drug products. Substantial new provisions affecting compliance have also been enacted, which may affect our business practices with health care practitioners. We will not know the full effects of the Health Care Reform Law until applicable federal and state agencies issue regulations or guidance under the new law. Although it is too early to determine the effect of the Health Care Reform Law, the new law appears likely to continue the pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA's approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
RISKS RELATING TO EMPLOYEE MATTERS AND MANAGING GROWTH
Our future success depends on our ability to retain our chief executive officer and other key executives and to attract, retain and motivate qualified personnel.
We are highly dependent on our management team, specifically Dr. Julian Kenyon and Mr. James Nathanielsz. While we have a current employment agreement with our CEO, Mr. James Nathanielsz and Dr. Julian Kenyon has a letter of appointment, both the employment agreement with Mr. Nathanielsz, and the letter of appointment for Dr. Kenyon, permit each of the respective parties thereto to terminate such agreements upon notice. As such, each of these individuals may terminate their relationship with us upon notice. If we lose key employees, our business may suffer.
23
Recruiting and retaining qualified scientific, clinical, manufacturing and sales and marketing personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors, including our scientific co-founders, may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.
We expect to expand our development, regulatory and future sales and marketing capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of drug development, regulatory affairs and sales and marketing. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The physical expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
We do not have any independent directors and there is a potential conflict of interest
Since we do not have an audit or compensation committee comprised of independent directors, the functions that would have been performed by such committees are performed by our directors, one of whom also serves as an officer of the Company. Thus, there is an inherent conflict of interest.
RISKS RELATED TO OUR COMMMON STOCK
Currently there is a limited public market for our common stock, and we cannot predict the future prices or the amount of liquidity of our common stock.
Currently, there is a limited public market for our common stock. Our common stock is listed on the Over-the-Counter Bulletin Board under the symbol "PPCH". However, the Bulletin Board is not a liquid market in contrast to the major stock exchanges. We cannot assure you as to the liquidity or the future market prices of our common stock if a market does develop. If an active market for our common stock does not develop, the fair market value of our common stock could be materially adversely affected. We cannot predict the future prices of our common stock.
If we do not comply with the state regulations in regard to the sale of our common stock or find an exemption there may be potential limitations on the resale of your stock.
With few exceptions, every offer or sale of a security must, before it is offered or sold in a state, be registered or exempt from registration under the securities, or blue sky laws, of the state(s) in which the security is offered and sold. Blue sky statutes vary widely and there is very little uniformity in the blue sky filing requirements among state securities laws. As of the date hereof, we intend to offer our common stock upon effectiveness of the registration statement of which this prospectus forms a part to potential purchasers in the states of New York, Florida, Massachusetts, Connecticut and Illinois. While we intend to review the relevant blue sky laws of these states before the distribution of the common stock therein, should we fail to properly register the common stock as required by the respective states or find an exemption from registration, you may not be able to resell your stock once purchased.
24
We are subject to the “penny stock” rules which will adversely affect the liquidity of our common stock.
The Securities and Exchange Commission, or the SEC, has adopted regulations which generally define “penny stock” to be an equity security that has a market price of less than $5.00 per share, subject to specific exemptions. We expect the market price of our common stock will be less than $5.00 per share and therefore we will be considered a “penny stock” according to SEC rules. This designation requires any broker-dealer selling these securities to disclose certain information concerning the transaction, obtain a written agreement from the purchaser and determine that the purchaser is reasonably suitable to purchase the securities. These rules limit the ability of broker-dealers to solicit purchases of our common stock and therefore reduce the liquidity of the public market for our shares should one develop.
Because directors and officers currently and for the foreseeable future will continue to control Propanc, it is not likely that you will be able to elect directors or have any say in the policies of Propanc.
Our shareholders are not entitled to cumulative voting rights. Consequently, the election of directors and all other matters requiring shareholder approval will be decided by majority vote. The directors and officers of Propanc beneficially own approximately 12.22% of our outstanding common stock. Due to such significant ownership position held by our insiders, new investors may not be able to effect a change in our business or management, and therefore, shareholders would have no recourse as a result of decisions made by management.
In addition, sales of significant amounts of shares held by our officer and directors, or the prospect of these sales, could adversely affect the market price of our common stock. Management’s stock ownership may discourage a potential acquirer from making a tender offer or otherwise attempting to obtain control of us, which in turn could reduce our stock price or prevent our stockholders from realizing a premium over our stock price.
In the future we may issue preferred stock without the approval of our shareholders, which could make it more difficult for a third party to acquire us and could depress our stock price.
Our board of directors may issue, without a vote of our shareholders, one or more series of preferred stock with such rights and preferences. This could permit our board of directors to issue preferred stock to investors who support us and our management and permit our management to retain control of our business. Additionally, issuance of preferred stock could block an acquisition which could result in both a drop in our stock price and a decline in interest of our common stock.
Since we intend to retain any earnings for development of our business for the foreseeable future, you will likely not receive any dividends for the foreseeable future, capital appreciation, if any, will be the source of gain for our stockholders.
We have never declared or paid any cash dividends or distributions on our capital stock. We currently intend to retain our future earnings to support operations and to finance expansion and therefore we do not anticipate paying any cash dividends on our common stock in the foreseeable future. As a result, capital appreciation, if any, of our common stock will be the sole source of gain for our stockholders for the foreseeable future.
A significant number of our shares will be eligible for sale and their sale or potential sale may depress the market price of our common stock.
Sales of a significant number of shares of our common stock in the public market could harm the market price of our common stock. This prospectus covers 171,000,000 shares of the Company's Common Stock, which underlie the Debenture. Assuming automatic conversion of the Debenture into Common Stock in connection with the completion of this offering, the 171,000,000 shares would represent approximately 29% of the issued and outstanding shares of our common stock. As additional shares of our common stock become available for resale in the public market pursuant to this offering, and otherwise, the supply of our common stock will increase, which could decrease its price. In addition some or all of the shares of common stock may be offered from time to time in the open market pursuant to Rule 144, and these sales may have a depressive effect on the market for our shares of common stock.
25
We will not receive any proceeds from the sale of shares by the Selling Security Holders. However, we received four tranches of proceeds from the sale of the Debenture and Warrant to the Purchaser for a principal amount of $4,400,000 (purchase price of $4,000,000 with a 10% original issue discount), pursuant to the Purchase Agreement, as modified by the Addendum. The four tranches received as of the date of this Registration Statement is as follows:
| Tranche | Gross Amount Received | Net Proceeds* | ||||||
| First Tranche | $ | 1,200,000 | $ | 1,150,000 | ||||
| Second Tranche | $ | 700,000 | 680,893 | |||||
| Third Tranche | $ | 525,000 | 510,670 | |||||
| Fourth Tranche | $ | 1,200,000 | 1,185,000 | |||||
| Totals | $ | 3,625,000 | 3,526,563 | |||||
*After professional fees
We expect to receive an additional tranche of funds, of $375,000, from the sale of a Debenture and Warrant pursuant to the Purchase Agreement, as modified by the Addendum. We have used the net proceeds from the sale of the Debenture and Warrant, and intend to use the net proceeds from the additional tranche yet to be released, for general corporate and working capital purposes and acquisitions or assets, businesses or operations or for other purposes that our board of directors (the “Board”), in its good faith deem to be in the best interest of the Company. The Company has agreed to bear the expenses relating to the registration statement for the shares underlying the Debenture and Warrant issued to the Selling Security Holder. Under the terms of the Debenture, the Company was entitled to a reduction in the principal amount of the October 2015 Financing of (i) $25,000 upon the Company’s filing on the Prior Registration Statement within the time period specified and (ii) $25,000 upon the effectiveness of the Prior Registration Statement within the time period specified. Both of these conditions were met resulting in a $50,000 reduction of the principal amount of the October 2015 Financing such that the current aggregate principal amount is $4,350,000 as of the date of this Registration Statement.
We could also receive up to $15,714,285.60
net of fees in the event the Warrant is exercised for cash (notwithstanding that such Warrant has a cashless exercise feature).
We will use the proceeds from the exercise of the Warrant for general corporate purposes, which may include, among other things,
our working capital needs and other general corporate purposes.
DETERMINATION OF OFFERING PRICE
The price at which the shares of Common Stock underlying the Debenture can be converted are determined based on such price or formula in the Purchase Agreement and Addendum entered into between the Company and such Selling Security Holders.
The selling security holder will offer common stock at the prevailing market prices or privately negotiated prices. The offering price of our common stock does not necessarily bear any relationship to our book value, assets, past operating results, financial condition or any other established criteria of value. Our common stock may not trade at market prices in excess of the offering price as prices for common stock in any public market will be determined in the marketplace and may be influenced by many factors, including the depth and liquidity.
There is not substantial disparity between the public offering price and the effective cash cost to officers, directors, promoters and affiliated persons of common equity acquired by them in transactions during the past five years and we were subject to the reporting requirements of section 13(a) and 15(d) of the Exchange Act immediately prior to filing the registration statement.
26
The shares of Common Stock being offered for resale by the selling shareholders consist of 171,000,000 shares of Common Stock underlying the Debenture.
The following table sets forth the names of the selling shareholders, the number of shares of Common Stock beneficially owned by each of the selling shareholders as of March 18, 2016 and the number of shares of Common Stock being offered by the selling shareholders. The shares being offered hereby are being registered to permit public secondary trading, and the selling shareholders may offer all or part of the shares for resale from time to time. However, the selling shareholders are under no obligation to sell all or any portion of such shares nor are the selling shareholders obligated to sell any shares immediately upon effectiveness of this prospectus. All information with respect to share ownership has been furnished by the selling shareholders.
| Common Stock | ||||||||||||||||||||
| Prior to the offering | After the offering | |||||||||||||||||||
| Selling Security Holder | Number of Shares of Common Stock Beneficially Owned | Percentage of Common Stock (1) | Shares being Offered | Number of Shares of common Stock Beneficially Owned | Percentage of Common Stock (1) | |||||||||||||||
| Delafield Investments Limited (2) | 171,000,000 | 29 | % | 171,000,000 | 0 | 0.00 | % | |||||||||||||
| Total | ||||||||||||||||||||
| (1) | Applicable percentage ownership is based on 584,531,788 shares of common stock outstanding as of March 18, 2016, assuming automatic conversion of the Debenture into common stock in connection with the completion of the offering. In computing the number of shares of common stock beneficially owned by the selling shareholder and the percentage ownership of such person, we deemed to be outstanding all shares of common stock subject to options, warrants and convertible notes currently exercisable or convertible, or exercisable or convertible within 60 days. However, we did not deem such shares outstanding for the purpose of computing the percentage ownership of any other person. | |
| (2) | Magna Gibraltar Investments LLC, a Delaware limited liability company, or Magna Gibraltar, is a partial owner of the Selling Stockholder and, through representation on the board of directors of the Selling Stockholder, controls the Selling Stockholder. Pursuant to a shareholders agreement relating to the ownership of the Selling Stockholder, the board of directors of the Selling Stockholder, acting by majority vote, has sole power to vote or to direct the vote and sole power to dispose or to direct the disposition of all securities owned directly by the Selling Stockholder, including, without limitation, the common stock issuable upon conversion of the Debenture and the exercise of the Warrant. The board of directors of the Selling Stockholder consists of three individuals, two of which are appointed by Magna Gibraltar. The two directors appointed by Magna Gibraltar are Joshua Sason and Michael Abitebol. |
This prospectus is to be used by the Selling Security Holder in connection with a potential resale by certain Seller Security Holder of up to an aggregate of 171,000,000 shares of the registrant’s Common Stock underlying the Debenture.
The common stock held by the selling stockholders may be sold or distributed from time to time by the selling stockholders directly to one or more purchasers or through brokers, dealers, or underwriters who may act solely as agents at market prices prevailing at the time of sale, at prices related to the prevailing market prices, at negotiated prices, or at fixed prices, which may be changed on any stock exchange, market or trading facility on which the shares are traded or in private transactions. The sale of the selling stockholders’ common stock offered by this prospectus may be effected in one or more of the following methods:
| ● | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| ● | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
27
| ● | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| ● | an exchange distribution in accordance with the rules of the applicable exchange; |
| ● | privately negotiated transactions; |
| ● | settlement of short sales entered into after the effective date of the Registration Statement of which this prospectus is a part; |
| ● | in transactions through broker-dealers that agree with the selling shareholders to sell a specified number of such shares at a stipulated price per share; |
| ● | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| ● | a combination of any such methods of sale; or |
| ● | any other method permitted pursuant to applicable law. |
The selling shareholders may also sell shares under Rule 144 under the Securities Act, if available, rather than under this prospectus.
In addition, the selling shareholders may enter into hedging transactions with broker-dealers who may engage in short sales, if short sales were permitted, of shares in the course of hedging the positions they assume with the selling shareholders. The selling shareholders may also enter into option or other transactions with broker-dealers that require the delivery by such broker-dealers of the shares, which shares may be resold thereafter pursuant to this prospectus. None of the selling shareholders are broker-dealers or affiliates of broker dealers. We will advise the selling shareholders that the anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of shares in the market and to the activities of the selling shareholders and their affiliates. In addition, we will make copies of this prospectus (as it may be supplemented or amended from time to time) available to the selling shareholders for the purpose of satisfying the prospectus delivery requirements of the Securities Act.
The selling shareholders may indemnify any broker-dealer that participates in transactions involving the sale of the shares against certain liabilities, including liabilities arising under the Securities Act.
Brokers, dealers, or agents participating in the distribution of the shares may receive compensation in the form of discounts, concessions or commissions from the selling shareholders and/or the purchasers of shares for whom such broker-dealers may act as agent or to whom they may sell as principal, or both (which compensation as to a particular broker-dealer may be in excess of customary commissions). Neither the selling shareholders nor we can presently estimate the amount of such compensation. We know of no existing arrangements between the selling shareholders and any other stockholder, broker, dealer or agent relating to the sale or distribution of the shares. We will not receive any proceeds from the sale of the shares of the selling shareholders pursuant to this prospectus. We have agreed to bear the expenses of the registration of the shares, including legal and accounting fees, and such expenses are estimated to be approximately $15,000.
Notwithstanding anything set forth herein, no FINRA member will
charge commissions that exceed 8% of the total proceeds of the offering.
28
Authorized Capital and Preferred Stock
Our authorized capital stock consists of 2,000,000,000 shares of common stock, par value $0.001 per share and 10,000,000 shares of preferred stock, par value $0.01 per share. As of March 18, 2016, there were 584,531,788 shares of common stock outstanding.
Common Stock
The following is a summary of the material rights and restrictions associated with our common stock.
Each share of Common Stock shall have one (1) vote per share for all purposes. Our Common Stock does not provide preemptive, subscription or conversion rights and there are no redemption or sinking fund provisions or rights. Holders of shares of Common Stock are not entitled cumulative voting for electing members of the Board. Please refer to the Company’s Articles of Incorporation, Bylaws and the applicable statutes of the State of Delaware for a more complete description of the rights and liabilities of holders of the Company’s securities.
Preferred Stock
Of the 10,000,000 shares of preferred stock authorized, 500,001 are issued and outstanding as follows: (i) 500,000 shares designated as Series A Preferred Stock; and (ii) 1 share designated as Series B Preferred Stock.
Holders of Series A Preferred Stock are entitled to vote on all matters submitted or required to be submitted to a vote of the shareholders, except election and removal of directors, and each holder shall be entitled to Five Hundred (500) votes per share of Series A Preferred Stock.
Holders of Series B Preferred Stock are entitled to voting power equivalent of the number of votes equal to the total number of Company’ common stock outstanding as of the record date for the determination of stockholders entitled to vote at each meeting of stockholders of the Company and entitled to vote on all matters submitted or required to be submitted to a vote of the stockholders of the Company.
Dividends
We have not paid any cash dividends to our shareholders. The declaration of any future cash dividends is at the discretion of our Board and depends upon our earnings, if any, our capital requirements and financial position, and general economic conditions. It is our present intention not to pay any cash dividends in the foreseeable future, but rather to reinvest earnings, if any, in our business operations.
Warrants
In September, 2013, pursuant to convertible debenture, the Company issued 3,000,000 warrants to purchase common stock. These warrants have an initial exercise price of $0.0698 per share which is subject to adjustment and expire 5 years from the date of issuance.
On May 7, 2015, the Company issued to a consultant, 3,379,158 warrants to purchase common stock. These warrants have an exercise price of $0.03 per share and expire 5 years from the date of issuance.
On May 21, 2015, the Company issued to a consultant, 1,000,000 warrants to purchase common stock. These warrants have an exercise price of $0.07 per share and expire 5 years from the date of issuance.
On October 28, 2015, pursuant to a Purchase Agreement entered into with Delafield Investments Limited, the Company issued warrants to purchase an aggregate of 26,190,476 shares of the Company’s common stock, par value $0.001 per share, for an exercise price of $0.60 per share for a period of four (4) years from the Closing Date (the “Delafield Warrant”). The Delafield Warrant is described in more detailed above under “Magna Financing”.
29
Convertible Debentures
On October 28, 2015, pursuant to a Purchase Agreement entered into with Delafield Investments Limited, the Company issued a 5% Original Issue Discount Senior Secured Convertible Debenture (the “Debenture”) in the principal amount of $4,400,000 (which includes a 10% original issue discount). On March 11, 2016, the Company entered into an Addendum with the Purchaser pursuant to which the Company and the Purchaser agreed to certain new terms with respect to the transactions contemplated by Purchase Agreement (the “Addendum”). The description of the Delafield Debenture below reflects the terms of the Debenture as modified by the Addendum.
Under the terms of the Debenture, the Company was entitled to a $50,000 reduction of the principal amount of the October 2015 Financing due to the satisfaction of certain conditions set forth therein. As a result, the current aggregate principal amount is $4,350,000 as of the date of this Registration Statement (the “Principal Amount”).
The Debenture has a 10% original issue discount and matures on October 28, 2016. The Principal Amount of the Debenture accrues interest at the rate of 5% per annum, payable quarterly in cash (or if certain conditions are met, in stock at the Company’s option) on January 1, April 1, July 1 and October 1. The Debenture is convertible at any time, in whole or in part, at the Purchaser’s option into shares of the Company’s common stock at a conversion price equal to $0.03 (the “Conversion Price”); provided that in the event that the volume weighted average price per share on any trading day is less than such conversion price, the conversion price will be adjusted to a price per share that is equal to a 22.5% discount to the lowest trading price of the common stock in the 10 trading days prior to the date of conversion. At no time will the Purchaser be entitled to convert any portion of the Debenture to the extent that after such conversion, the Purchaser (together with its affiliates) would beneficially own more than 4.99% of the outstanding shares of Common Stock as of such date. The Delafield Debenture is described in more detailed above under “Magna Financing”.
Options
There are no outstanding options to purchase our securities.
Anti-takeover provisions under our charter documents and Delaware law could delay or prevent a change of control which could limit the market price of our common stock and may prevent or frustrate attempts by our stockholders to replace or remove our current management.
Our amended and restated certificate of incorporation and amended and restated bylaws contain provisions that could delay or prevent a change of control of our company or changes in our board of directors that our stockholders might consider favorable. Some of these provisions include:
| • | a requirement that all shareholder actions require written notice by holders of at least 10% of the shares entitled to vote at the meeting; |
| • | advance notice requirements for stockholder proposals and nominations for election to our board of directors; |
| • | a requirement that no member of our board of directors may be removed from office by our stockholders except for cause and, in addition to any other vote required by law, upon the approval of not less than 66-2/3% of all outstanding shares of our voting stock then entitled to vote in the election of directors; |
| • | the authority of the board of directors to issue preferred stock on terms determined by the board of directors without stockholder approval and which preferred stock may include rights superior to the rights of the holders of common stock. |
In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporate Law, which may prohibit certain business combinations with stockholders owning 15% or more of our outstanding voting stock. These anti-takeover provisions and other provisions in our amended and restated certificate of incorporation and amended and restated bylaws could make it more difficult for stockholders or potential acquirers to obtain control of our board of directors or initiate actions that are opposed by the then-current board of directors and could also delay or impede a merger, tender offer or proxy contest involving our company. These provisions could also discourage proxy contests and make it more difficult for you and other stockholders to elect directors of your choosing or cause us to take other corporate actions you desire. Any delay or prevention of a change of control transaction or changes in our board of directors could cause the market price of our common stock to decline.
30
Transfer Agent and Registrar
Corporate Stock Transfer, Inc., 3200 Cherry Creek South Drive, Suite 430, Denver, CO. 80209, Telephone: 303-282-4800, Fax: 303-282-5800.
Listing
The shares of our common stock are quoted on the OTCQB under the symbol PPCH. On March 17, 2016, the last reported sale price per share for our common stock on the OTCQB as reported was $0.03.
INTERESTS OF NAMED EXPERTS AND COUNSEL
No expert or counsel named in this prospectus as having prepared or certified any part of this prospectus or having given an opinion upon the validity of the securities being registered or upon other legal matters in connection with the registration or offering of the Common Stock was employed on a contingency basis, or had, or is to receive, in connection with the offering, a substantial interest, direct or indirect, in the registrant or any of its parents or subsidiaries. Nor was any such person connected with the registrant or any of its parents or subsidiaries as a promoter, managing or principal underwriter, voting trustee, director, officer, or employee.
The consolidated financial statements for the years ended June 30, 2015 and 2014 included in this prospectus and the registration statement have been audited by Salberg & Company, P.A. to the extent and for the periods set forth in their report appearing elsewhere herein and in the registration statement, and are included in reliance upon such report given upon the authority of said firm as experts in auditing and accounting.
The validity of the issuance of the Common Stock hereby will be passed upon for us by Szaferman Lakind Blumstein & Blader, PC, Lawrenceville, New Jersey.
Overview
We are a development stage healthcare company that is currently focused on developing new cancer treatments for patients, suffering from pancreatic, ovarian and colorectal cancers. Together with our scientific and oncology consultants, we have developed a rational, composite formulation of anti-cancer compounds, which together exert a number of effects designed to control or prevent tumors from recurring and spreading through the body. Our leading products are variations upon our novel formulation and involve or employ pro-enzymes, which are inactive precursors of enzymes. As a result of positive early indications of the anti-cancer effects of our technology, we intend to submit our pro-enzyme treatment to the rigorous, formal non-clinical and clinical development and trial processes required to obtain the regulatory approval necessary to commercialize it and any product(s) derived and/or to be derived therefrom.
In the near term, we intend to target patients with limited remaining therapeutic options for the treatment of solid tumors such as pancreatic, ovarian and colorectal tumors. In the future, we intend to development our lead product to treat (i) early stage cancer and (ii) pre-cancerous diseases and (iii) as a preventative measure for patients at risk of developing cancer based on genetic screening.
Key Highlights of this opportunity are:
| · | Potential cancer treatment: a once-daily pro-enzyme treatment as a clinically proven therapeutic option in cancer treatment and prevention. |
| · | Multiple mechanisms of action on cancerous or carcinogenic cells: our treatment exerts multiple effects on cancerous cells which inhibits tumor growth and potentially stop the tumor from spreading through the body in contrast to cancer treatments currently available that lack sufficient efficacy to achieve a durable clinical response by preventing tumor recurrence, or inhibiting new growths which spread through the body. As we progress our research, we intend to elucidate further the multiple mechanisms of action to identify opportunities to expand our intellectual property portfolio. Furthermore, we hope to uncover the molecular targets of the pro-enzymes to identify potential opportunities for developing new compounds. |
31
| · | Encouraging data from patient treatment: Scientific research undertaken over the last 15 years and clinical experience from treating patients in the United Kingdom (the “UK”) and Australia has provided evidence that PRP may be an effective treatment against cancer and warrants further development. | |
| · | Unique intellectual property: We are focusing on building a significant portfolio of intellectual property around the use of pro-enzymes in the treatment of cancer, identifying new formulations, alternative routes of administration and potential new therapeutic targets. The PRP drug product is an enhanced pro-enzyme formulation comprising amylase and pro-enzymes of trypsinogen and chymotrypsinogen, in a specific ratio which synergistically enhances the anti-cancer effects of the pro-enzymes compared to when used as singular entities. Patent protection is currently being sought for this PRP drug product, which forms part of the subject matter of International (PCT) Patent Application No. PCT/AU2010/001403 filed on October 22, 2010 in the name of Propanc Pty Ltd. This international PCT application also includes the priority filings of Australian provisional patent application # 2009905147 and # 2010902655, which were filed on October 22, 2009 and June 17, 2010 respectively (as discussed under the section “Intellectual Property”). The PRP-DCM drug product also forms part of the subject matter of International (PCT) Patent Application No. PCT/AU2010/001403. The Authorized Officer indicated in the Written Opinion issued for this international PCT application, that the patent claims covering the PRP and PRP-DCM products were novel over the prior art cited in the International Search Report. Various national phase applications are being filed in countries around the world based on the above priority applications. More recently, an Australian provisional patent application #2015904678 was filed on November 12, 2015, relating to proenzyme compositions and on January 29, 2016, a Spanish provisional application #201630112 was filed citing a method to treat cancer by eradicating cancer stem cells whilst sparing normal stem cells. |
| · | Market opportunity: Growing demand for new cancer treatments as a result of a rapidly aging population and changing environmental factors in western countries. According to the World Health Organization, all cancers (excluding non-melanoma skin cancer) are expected to increase from 8.2 million annual deaths in 2012 to over 10 million annual deaths by 2020, exceeding 13 million annual deaths by 2030. |
Magna Financing
On October 28, 2015 (the “Closing Date”), Propanc Health Group Corporation, a Delaware corporation (the “Company”), entered into a securities purchase agreement dated as of the Closing Date (the “Purchase Agreement”) with Delafield Investments Limited (the “Purchaser”). The Purchase Agreement provides that, upon the terms and subject to the conditions set forth therein, the Purchaser will invest $4,000,000 (“Investment Amount”) in exchange for a Convertible Debenture (the “Debenture”) in the principal amount of $4,400,000 (the “Principal Amount”) and warrants to purchase an aggregate of 26,190,476 shares of the Company’s common stock, par value $0.001 per share, for an exercise price of $0.60 per share for a period of four (4) years from the Closing Date (the “Warrant”). Pursuant to the Purchase Agreement, on the Closing Date, the Company issued the Debenture and Warrant to the Purchaser.
On March 11, 2016, the Company entered into an Addendum with the Purchaser pursuant to which the Company and the Purchaser agreed to certain new terms with respect to the transactions contemplated by Purchase Agreement (the “Addendum”). The description of the Debenture below reflects the terms of the Debenture as modified by the Addendum.
The Company filed a registration statement on Form S-1 on November 23, 2015, which was deemed effective on December 10, 2015, pursuant to which the Company previously registered an aggregate of 98,404,985 shares of the Company's Common Stock consisting of: (i) 72,214,509 shares underlying the Debenture; and (ii) 26,190,476 shares of Common Stock issuable upon exercise of the Warrant (the “Prior Registration Statement”).
Under the terms of the Debenture, the Company was entitled to a reduction in the principal amount of the October 2015 Financing of (i) $25,000 upon the Company’s filing on the Prior Registration Statement within the time period specified and (ii) $25,000 upon the effectiveness of the Prior Registration Statement within the time period specified. Both of these conditions were met resulting in a $50,000 reduction of the principal amount of the October 2015 Financing such that the current aggregate principal amount is $4,350,000 as of the date of this Registration Statement (the “Principal Amount”). Any references to the “principal amount” or the defined term “Principal Amount” used in this Registration Statement shall refer to the reduced Principal Amount as described herein.
32
Debenture
The Debenture has a 10% original issue discount and matures on October 28, 2016. The Principal Amount of the Debenture accrues interest at the rate of 5% per annum, payable quarterly in cash (or if certain conditions are met, in stock at the Company’s option) on January 1, April 1, July 1 and October 1. The Debenture is convertible at any time, in whole or in part, at the Purchaser’s option into shares of the Company’s common stock at a conversion price equal to $0.03 (the “Conversion Price”); provided that in the event that the volume weighted average price per share on any trading day is less than such conversion price, the conversion price will be adjusted to a price per share that is equal to a 22.5% discount to the lowest trading price of the common stock in the 10 trading days prior to the date of conversion. At no time will the Purchaser be entitled to convert any portion of the Debenture to the extent that after such conversion, the Purchaser (together with its affiliates) would beneficially own more than 4.99% of the outstanding shares of Common Stock as of such date.
Warrant
The Warrant is exercisable in whole or in part, at an initial exercise price per share of $0.60, subject to adjustment. The exercise price and number of shares of the Company’s common stock issuable under the Warrant are subject to adjustments for stock dividends, splits, combinations, subsequent rights offerings and pro rata distributions. Any adjustment to the exercise price shall similarly cause the number of warrant shares to be adjusted so that the total value of the Warrant may increase. In the event that the shares underlying the Warrant are not included in an effective registration statement, the Warrant may be exercised on a cashless basis.
Registration Rights Agreement
In connection with the execution of the Purchase Agreement, on the Closing Date, the Company and the Purchaser also entered into a registration rights agreement dated as of the Closing Date (the “Registration Rights Agreement”). Pursuant to the Registration Rights Agreement, the Company agreed to file an initial registration statement (“Registration Statement”) with the SEC to register the resale of the Common Stock into which the Debenture may be converted or the Warrant may be exercised. Such Registration Statement was filed on November 23, 2015 and declared effective on December 10, 2015 (the “Prior Registration Statement”). An aggregate of 98,404,985 shares of the Company's Common Stock were registered pursuant to the Prior Registration Statement consisting of: (i) 72,214,509 shares underlying the Debenture; and (ii) 26,190,476 shares of Common Stock issuable upon exercise of the Warrant.
Security Agreement
In connection with the Purchase Agreement, the Company entered into a Security Agreement dated as of even date therewith with the Purchaser whereby the Company agreed to grant to Purchaser an unconditional and continuing, first priority security interest in all of the assets and property of the Company to secure the prompt payment, performance and discharge in full of all of Company’s obligations under the Debenture, Warrant and the other transaction documents until ten days following the such time as the Registration Statement is declared effective by the SEC and the equity conditions set forth in the Debenture are met.
Company History
Propanc Health Group Corporation, formerly Propanc PTY Ltd., is a development stage enterprise incorporated in Melbourne, Victoria Australia on October 15, 2007. Based in Melbourne, Victoria Australia, since inception, substantially all of the efforts of our company have been the development of new cancer treatments targeting high risk patients who need a long term therapy which prevents the cancer from returning and spreading. The Company anticipates establishing global markets for its technologies.
On November 23, 2010, Propanc Health Group Corporation was incorporated in the state of Delaware. In January 2011, to reorganize the Company, Propanc Health Group Corporation acquired all of the outstanding shares of Propanc PTY Ltd. on a one-for-one basis making it a wholly-owned subsidiary.
33
We were formed for the specific purpose of having shareholders of Propanc PTY Ltd. directly owning an interest in a U.S. company. On January 29, 2011, we issued 64,700,525 shares of our common stock in exchange for 64,700,525 shares of Propanc PTY Ltd. common stock.
Propanc’s scientific roots date back almost 100 years to the work of Professor John Beard at the University of Edinburgh in the United Kingdom (the “UK”) whose pioneering work on tumor cell biology and potential new approaches to treating cancer by targeting specific pathways which kill off cancer cells, but leave healthy cells alone. In more recent times interest in the work of Professor Beard has re-emerged, driven by the insights into his work offered with modern day knowledge of tumor cell and molecular biology.
Important Milestones for Propanc
| · | From the late 90’s, work from other scientists and clinicians, including Dr. Josef Novak in the U.S. and a since retired oncologist, Dr. Frantisek Trnka, from the Czech Republic, shed new light on the therapeutic potential of Professor Beard’s insights. Extensive laboratory work undertaken over a number of years by Novak and Trnka was reported in the journal Anticancer Research in 2005 in the paper entitled ‘Pro-enzyme Therapy of Cancer’. The conclusion of Novak and Trnka from this work was the discovery “that pro-enzyme therapy mandated first by John Beard nearly one hundred years ago, shows remarkable selective effects that result in growth inhibition of tumor cells with metastatic potential”. Today, these important scientific observations support our view that pro-enzymes are selective and effective in targeting malignant tumor cells and could become an effective tool in the fight against metastatic cancer. |
| · | In 2007, Dr. Julian Kenyon, Medical Director of the Dove Clinic in the United Kingdom and Dr. Douglas Mitchell, further developed the therapeutic concepts of Beard and identified strategies which could improve upon the therapeutic potential of Beard’s original ground-breaking work. A suppository formulation was developed by Mandeville Medicines, Buckinghamshire, UK, at the request of, and in consultation with, Dr. Kenyon and Dr. Mitchell, in an effort to improve on results reported in the literature pertaining to the potential therapeutic use of pro-enzymes in cancer treatment. Patients were first treated with the suppository formulation in April 2007 at The Dove Clinic, UK and in July 2007 at the Opal Clinic, Australia. Drs. Kenyon and Mitchell, through The Dove Clinic and Opal Clinic respectively, treated cancer patients in the United Kingdom and Australia with a suppository formulation of pro-enzymes. The treatment was undertaken under special UK and Australian regulatory provisions. In the UK it was undertaken under the Medicines and Healthcare Products Regulatory Agency (the “MHRA”)’s regulations designed for patients who have special clinical needs that cannot be met by licensed medicinal products, and in Australia under the Therapeutic Goods Administration or TGA Special Access Scheme, a mechanism which provides for the import and/or supply of an unapproved therapeutic good for a single patient, on a case by case basis. In both jurisdictions, patients are permitted to receive treatment on an individual basis for compassionate use as long it is supplied by a recognized, licensed manufacturer who is able to meet certain guidelines for unapproved products, and individual case files are maintained for patients should the regulatory authorities require this information. No prior approval was required by either the MHRA or TGA prior to the commencement of treatment. No suppository formulation of the pro-enzymes was available and it was necessary for a novel suppository formulation to be manufactured specifically for these patients by a suitably licensed manufacturer. |
| · | Forty-six late stage cancer patients suffering from a range of malignancies in the UK and Australia received treatment with the pro-enzyme suppositories over periods of time ranging from one (1) month to in excess of seventeen (17) months. Inspired by their observations in clinical practice, Dr. Kenyon and Dr. Mitchell resolved to develop pro-enzyme therapy for cancer patients worldwide. |
| · | Late 2007, Dr. Kenyon, Dr. Mitchell and Mr. James Nathanielsz, our Chief Executive Officer, developed a strategy to commercialize the newly developed pro-enzyme formulation, now designated PRP. Propanc Pty Ltd was established in Australia to refine, develop and commercialize novel, patented pro-enzyme therapeutics for the treatment of cancer. This remains our intention to date. |
34
| · | In 2008, a Scientific Advisory Board (the “Advisory Board”) comprising Professor John Smyth (Edinburgh University), Professor Klaus Kutz (Bonn University) and Professor Karrar Khan (De Montfort University) was established. Dr Ralf Brandt, Chief Executive Officer and Founder of preclinical Contract Research Organization (CRO), vivoPharm Pty Ltd., was later appointed to the Board in 2011. Today, the expertise of the Advisory Board in oncology research and development will be relied upon as we initiate patient trials and advance our products down the requisite regulatory pathways to commercialize our pro-enzyme therapies. |
| · | In 2009, a retrospective review of the patient notes from the forty-six (46) patients treated was undertaken by Dr. Kenyon. This report was subject to analysis by Professor Klaus Kutz who, at the time of the review, was an independent consultant in clinical pharmacology and safety, specializing in oncology. Professor Kutz observed that no patients were reported as living for a period less than that predicted by the treating clinician and a number of terminally ill patients lived marginally longer than predicted, particularly those suffering from pancreatic, colorectal, ovarian and gastro-intestinal cancers. As a result of the observations made by Dr. Kenyon and Professor Kutz, we are targeting the development of pro-enzyme therapy for the treatment of colorectal and pancreatic cancers for clinical trials, and in the future targeting other cancer types as our product candidate progresses to commercialization. |
| · | In early 2008, a research collaborative partnership was established with Professor David Tosh, at the Center for Regenerative Medicine, Deparment of Biology and Biochemistry, Bath University, to investigate the molecular mechanisms by which the pro-enzyme formulation is acting, which resulted in us filing two provisional patents a year later. We undertook additional scientific research with Professor Tosh, Dr. Macarena Pèran, Department of Health Sciences, Jaén University, and Dr. Juan Antonio Marchal, Biopathology and Regenerative Medicine Institute, Granada University. Important anti-cancer effects of the pro-enzymes were discovered, including triggering cell necrosis (cell death) and apoptosis (programmed cell death) and significantly, the induction of cell differentiation (i.e. inducing cancer cells to exhibit normal cell behavior). This led to us increasing our intellectual property base and patent new pharmaceutical compositions designed to enhance the effects of pro-enzymes. Subsequently, two provisional patents were combined into one Patent Cooperation Treaty (PCT) Application, filed on 22 October 2010 (PCT Application), and then a year later, we completed a 30 month national phase filing deadline for an international patent and commenced entering the national phase in countries around the world. So far, we received grant status in South Africa and more recently in Australia and New Zealand. In addition, the United States Patent and Trademark Office or USPTO and European Patent Office or EPO have made preliminary indications that key features of our technology are patentable. We are presently working towards securing a patent in each region, covering as many aspects of its technology as possible, whilst also actively seeking protection throughout Eastern Europe, Asia and South America. |
| · | Late 2010, we made additional important discoveries and scientific observations, resulting in additional composition claims which were included in the PCT Application, further protecting the company’s pro-enzyme formulation. Collaboration with vivoPharm Pty Ltd. (vivoPharm), located in Melbourne, Australia, with research facilities in Hershey, Pennsylvania, United States, identified a highly synergistic ratio of the pro-enzymes when combined together, resulting in increased anti-cancer effects in several tumor cell lines. By 2011, further work completed by vivoPharm confirmed the anti-metastatic effects of the newly combined ratio of the pro-enzymes in various cell line assays, and anti-angiogenic (inhibition of blood vessel formation) properties of the pro-enzyme treatment in mice. |
| · | In mid-2012, we began trading on the Over the Counter Bulletin Board (“OTCBB”) and are currently trading on OTCQB under OTC Markets. At the time, whilst located in Melbourne, Australia, we decided to access the US capital markets for raising the capital needed to finance the company’s pro-enzyme treatment for future clinical trials. Today, after deepening our scientific knowledge of the anti-cancer effects of pro-enzymes through our ongoing efforts with our research partners and strengthening our intellectual property portfolio by filing our patents in countries around the world, we are ready to complete the formal animal studies necessary to undertake human trials in 2016. |
| · | In May 2013, it was observed that pro-enzymes enforce the re-entry of cancer cells back into normal cellular pathways and this may represent a novel approach to the treatment of cancer. These findings were published in Cellular Oncology, a peer reviewed journal of the International Society for Cellular Oncology. |
35
| · | In 2014, after conducting a detailed strategic review of our scientific and preclinical research, our development team determined parenteral administration as the preferred route for the Company’s lead product, PRP. This approach would be the best way to maximize results in future patient trials, by ensuring maximum exposure of the drug to the tumor site. |
| · | Mid 2015, Dr. Joseph Chalil joined Propanc’s Scientific Advisory Board as an independent expert to provide advice on the Company’s drug development programs, in particular, Propanc’s lead product, PRP. Dr. Chalil is a Physician and Executive at Boehringer Ingelheim, the world's largest privately held pharmaceutical company. |
| · | In July 2015, a joint research collaboration agreement is established with Adaptive Biotechnologies in Seattle, WA, a leader in immune-sequencing, who will assist in determining a patient’s immune response post treatment with PRP. By studying the effects of the immune response post treatment, we plan to more accurately predict patient populations most likely to respond to PRP, as well as explore ways to further enhance the patient’s immune response during the treatment process. |
| · | Between July 2015 and February 2016, several scientific research findings are announced demonstrating significant anti-tumor efficacy in several animal models, including pancreatic and ovarian cancers at higher doses when administering proenzymes by intravenous injection, dramatic suppression of cancer stems cells in cell culture by altering several key pathways involved with invasion and metastasis, and identification of a synergistic response in a broad range of cancer types including kidney, melanoma, brain, prostate, liver, uterine and lung cancers. |
The Problem
In the early phases of tumor progression, cancer cells multiply near the site where their predecessors first began uncontrolled proliferation. The result, usually over a long period of time, is a primary tumor mass. Tumors often need to reach a large size before they make themselves apparent to the individual concerned, or the clinician screening for them.
Eventually, tumors of substantial size may begin to compromise the functioning of organs in which they have arisen and begin to evoke symptoms. In many cases, the effects on normal tissue function come from the physical pressure exerted by the expanding tumor masses. For example, large tumors in the colon may obstruct digestion products through the lumen, or in the lungs, airways may be compromised.
As dangerous and threatening as these primary tumors are, they are ultimately responsible for only about 10% of deaths. A far greater threat often arises for the patient, even after a primary tumor has been identified and removed. This threat involves cancerous growths that are discovered at sites far removed from the locations in their bodies where their primary tumors first appeared. These cancerous growths, called metastases, are responsible for 90% of patient deaths from cancer. Metastases are formed by cancer cells that have left the primary tumor mass and traveled by the body’s blood and lymphatic vessels (a vein like vessel carrying lymph, or white blood cells, from the tissues) to seek new sites and form new colonies. For example, breast cancers often spawn metastatic colonies in many tissues throughout the body including the brain, liver, bones, and lungs.
For primary tumors which have not yet metastasized, current treatments for cancer can be effective in initially reducing tumor burden. However, for many forms of cancer, current treatments lack sufficient efficacy to achieve a long lasting clinical response. Therefore, a vast majority of patients who succumb to cancer are killed by tumors that have metastasized. Continuing with the example of breast cancer, according to the National Cancer Institute’s SEER Cancer Statistics Review (2001 – 2007), of the patients diagnosed with late stage metastatic breast cancer, only 23% are expected to live longer than five years. This is compared to a 98% five year survival rate for an early stage breast cancer patient when the cancer is confined to the primary site.
The invasion-metastasis cascade
The great majority of life threatening cancers occur in epithelial tissues, yielding carcinomas. Epithelial cells generally have a multi-sided, uniform shape. They have well defined contact points with neighboring cells and a strong attachment to the underlying connective tissue, or stroma, which creates a framework for solid tumors in the body. Separating the two is the specialized type of extracellular matrix, known as the basement membrane.
By definition, carcinomas which originate on the epithelial side of the basement membrane and are considered to be benign, as long as the cells forming them remain on the same side. However, many carcinomas acquire the ability to penetrate the basement membrane, and individual cancer cells or groups of cancer cells begin to invade the stroma. This mass of cells is now reclassified as malignant. Often, many pathologists and surgeons reserve the label “cancer” for those epithelial tumors that have acquired this invasive ability.
36
Thereafter, carcinoma cells may invade into lymphatic or blood microvessels. The latter may then transport these cancer cells to distant sites in the body where they may be trapped and subsequently form new metastases.

It is important to note, that even before cells penetrate the basement membrane, they often stimulate angiogenesis (blood vessel formation) on the stromal side of the membrane, by expressing angiogenic proteins through the porous barrier. Not only does this enhance the ability of malignant cells to circulate into the blood, but also provides an important feedback loop for the cancer cell to maintain its invasiveness.
Understanding the mechanism by which benign cells change to a malignant state is therefore pivotal to developing anti-cancer treatments that have sufficient efficacy to achieve a long lasting clinical response.
The epithelial-mesenchymal transition and associated loss of E-cadherin expression enable carcinoma cells to become invasive.
Epithelial cells can undergo a transformation to a different cell type, called mesenchymal cells, through a process called the epithelial-to-mesenchymal transition, or EMT. Mesenchymal cells have an elongated spindle shape; lack orderly contacts with neighboring cells and can survive without contact with a surface or connective tissue. The EMT process is a series of events that normally occur during the development of tissues and organs prior to birth, and also apply to normal wound healing processes. However, the same EMT process can also be applied to epithelial cancer cells, or carcinomas. When epithelial carcinoma cells residing in a solid tumor undergo the EMT process, the resulting mesenchymal cancer cells can invade through local barriers and metastasize to other parts of the body.
In addition to becoming invasive and motile after undergoing the EMT process, the resulting mesenchymal cells have significantly increased resistance to current cancer treatments. For example, in Cancer Research in 2005, it was reported that lung cancer cells expressing mesenchymal biomarkers appeared to be resistant to Tarceva and other targeted anti-cancer agents when transplanted into mice.
37
At the center of this critical process for transforming benign cells into carcinomas, is the protein Epithelial Cadherin, or E-Cadherin. In normal cells, E-cadherin is located in the membrane and involved in maintaining cell to cell contact, which is critical to normal function and structure of epithelial tissues. The individual E-Cadherin molecules are attached to the actin (scaffolding, or cytoskeleton structure) within the cell, anchored by β-catenin, a protein which helps form the junction between epithelial cells. As well as forming an anchor between epithelial cells, β-catenin is also involved in gene transcription, a process by which DNA (deoxyribose nucleic acid) is converted into RNA (ribose nucleic acid) within the nucleus of a cell for the purpose of producing new proteins normally associated with routine cell function.
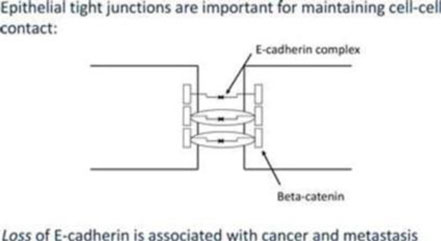
In the case of tumors, when cells become invasive, E-Cadherin expression decreases substantially, β-catenin becomes free within the cell, which may then migrate to the nucleus and induce expression of the EMT program. Furthermore, once cells undergo an EMT, they begin to produce their own cytokines (cell signaling molecules), such as Transforming Growth Factor β, or TGF-β. This protein plays a critical multi-functional role in promoting angiogenesis, immunosuppression (suppressing the immune system from recognizing and attacking cancer cells), and maintaining their mesenchymal cell structure for prolonged periods via a feedback mechanism. Studies also suggest that TGF-β works with β-catenin to cause epithelial cancer cells to undergo an EMT.
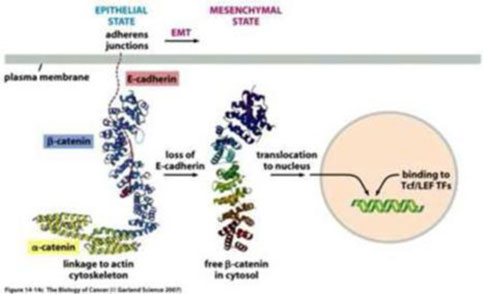
38
A study in the British Journal of Cancer, 2011, demonstrated that in cholangiocarcinoma (bile duct cancer) cell lines, treatment of TGF-β increased cell migration, invasion and mesenchymal changes. Furthermore, expression of E-cadherin and N-cadherin was measured from resected (cut out) specimens from extra-hepatic (outside the liver) cholangiocarcinoma patients. Patients with low E-cadherin expression had a significantly lower survival rate than patients with high E-cadherin expression. They concluded the cadherin switch via TGF-β induced EMT in extra-hepatic cholangiocarcinoma leads to cancer progression.
Conversely, in studies of several types of carcinoma cells that had lost E-cadherin expression, re-expression of this protein strongly suppressed the invasiveness and motility of these cancer cells.
Together, these observations indicate that E-Cadherin levels is a key determinant of the biological behavior of epithelial cancer cells and that the cell to cell contact constructed by E-cadherin molecules impede invasiveness and hence metastasis.
Our Solution
Our solution is to develop and commercialize a long-term therapy to prevent tumor recurrence and metastases, the main cause of patient death from cancer. We believe this problem can be addressed by developing a pro-enzyme formulation specifically targeting malignant carcinoma cells to and create a long lasting clinical benefit to the patient.
Propanc’s Theory Pro-enzymes Regulate Cell Proliferation
More than 100 years ago, Professor Beard, a comparative embryologist, made an observation that the pancreas develops in most vertebrates at the time when the placenta begins to slow its rate of growth. He hypothesized that enzymes produced by the developing pancreatic gland curtail trophoblastic invasion (a rare condition in which abnormal cells grow inside the uterus from tissue that forms after conception) and suggested that pancreatic extracts should have a similar inhibitory effect on invasive tumors.
Subsequently in the late 90’s, after following Professor Beard’s recommendations, Novak and Trnka hypothesized that administration of pro-enzymes, rather than the enzymes, was of crucial importance to the clinical effectiveness of the treatment approach first developed by Professor Beard, and that the precursor nature of the active enzymes may offer protection against numerous serpins (proteins which can inhibit pro-enzymes) in the blood.
As knowledge of tumor cell and molecular cell biology has increased over the years, our scientists and research partners have made important scientific discoveries identifying that pro-enzymes suppress the EMT program and induce cell differentiation, i.e., return cancerous cells towards normal cell behaviour, or a benign state.
After more than 100 years, the initial observations made by Professor Beard may have a potential common link between embryogenesis and cancer, by which cells are able to become motile and invasive, via the EMT program, where the administration of pro-enzymes may regulate cell proliferation as a means to controlling carcinomas.
Our Product Candidates
We are using our intellectual property and expertise to develop a pro-enzyme therapy for the treatment and prevention of the development of carcinomas from solid tumors. Initially, our products will be used in the treatment of pancreatic and colorectal cancers. In the future, we intend to expand our products scope in anti-cancer treatment to include other common solid tumors such as ovarian, gastrointestinal and prostate cancers.
PRP
Our lead product, PRP, is a novel, patented, formulation consisting of two pro-enzymes; trypsinogen and chymotrypsinogen, plus the enzyme amylase (1, 4-α-D-glucan glucanohydrolase). In limited human testing as described earlier, supplemented by laboratory research at the Universities of Bath and Granada on the mechanism of action of the pro-enzyme mixture, evidence has been obtained which suggests PRP may be effective against a range of solid tumors.
39
Selectivity
Research published in 2005, suggests that the pro-enzymes in our product, typsinogen and chymotrypsinogen exhibit specificity for tumor cells and not normal cells. Once activated, they in turn activate Protease Activated Receptors Type 2 (PAR2), which are located on the cell membrane and involved with cancer cell proliferation. Activation of PAR 2 results in a cascade of intracellular activities, including activation of a major component of the cell which controls its structure and architecture, the actin cytoskeleton. In a cancer cell, pro-enzymes have the effect of converting globular actin into filamentous actin, which causes the cell structure to collapse and induce cell death. This reduces tumor volume and is often seen in clinical practice.
In addition, the enzyme amylase contributes to the anti-tumor activity by splitting the carbohydrate element of glycoproteins on the surface of the tumor cell; this action is facilitated by the activated proteases around the cell.
Anti-Cancer Effects and Mechanism of Action
PRP consists of pro-enzymes which are known to influence a number of pathways critical for cancer cells to invade, grow and metastasize. Research published in 2013, shows the clinical benefits of PRP appear to result from enhanced differentiation of tumor cells, which inhibits proliferation and consequently, reduces their ability to invade and metastasize.
Specifically, we showed that pro-enzymes:
| · | induce a dose-dependent inhibition of cell growth, triggering apoptosis and cell necrosis; | |
| · | enhance expression of epithelial markers, such as E-cadherin and β catenin; | |
| · | decrease expression of EMT transcription factors responsible for coding specific gene sequences from DNA, associated with TGF-β cell signaling pathways; and | |
| · | induce malignant cells to differentiate to benign forms. |
Once activated, pro-enzymes influence the micro-immune environment around the cell, altering a number of pathways critical for supporting cancer cell growth, invasion and metastasis. This includes interacting with proteinases and cell signaling pathways in the extracellular matrix, whilst also interacting directly with cell surface proteins that effect the internal pathways of the cancer cell, triggering re-expression of epithelial markers, reducing important EMT markers, and inducing a series of cellular activities which alters the cancer cell’s morphology (structure) from a malignant to a benign state.
Preclinical Development
PRP activates E-Cadherin and β-Catenin Expression, Inhibiting Cell Growth in a Dose Dependent Manner
Initial experiments were performed to determine the effects of PRP on cell growth. Increasing doses of the pro-enzymes in PRP, trypsinogen and chymotrypsinogen were administered at increasing concentrations on three cancer-derived cell lines, including colorectal (Caco-2), pancreatic (Panc1) and esophageal (OE33) carcinomas.
Overall the cell numbers of these three cell lines slightly decreased at concentrations of ≤22 × 103 ng per mL for both trypsinogen and chymotrypsinogen, and ≤5.15 × 103 ng per mL for amylase. However, at ≤28 × 103 ng per mL for both trypsinogen and chymotrypsinogen, and ≤6.15 × 103 ng per mL for amylase, the cell numbers dropped sharply to below 60% and significantly decreased further at higher concentrations, especially for Caco-2 and OE33 carcinoma cell lines. These results suggest that PRP affects cellular growth in a dose dependent manner.
40
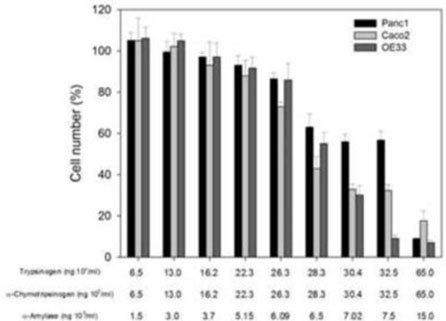
PRP increases the Expression of Epithelial Markers in Carcinomas
Upon treatment with PRP, changes in expression of the epithelial markers β-catenin and E-cadherin were assessed in Caco-2, Panc1 and OE33 cells. Subsequent flow cytometry analysis (flow cytometry is a laser-based technology that quantitate properties of single cells, one cell at a time) revealed that expression of E-cadherin in Caco-2 cells increased to 76.6% ± 3.0 when cells were treated with PRP, as compared to a control using untreated cells (15.9 % ± 4.2).
Changes in the expression of β-catenin in Caco-2 cells were also observed with an increase from 14.0 % ± 3.5 in control cells to 78.3% ± 0.3 after PRP treatment. E-cadherin expression increased to 42.3 %±6.1 and β-catenin to 47.1% ± 3.3 when Panc1 cells were treated with PRP, while in control cells the respective expression levels were 9.8 %± 2.9 and 34.7 %±7.4. Finally, PRP treated OE33 cells also showed an increment of both epithelial markers compared to untreated control cells, i.e., E-cadherin increased up to 52.4 %±6.8, whereas control cell expression was 8.4 %± 2.1, and β-catenin increased up to 30.2 %±4.2, whereas untreated control cells showed 8.0 %±0.7 expression. In all cases differences between untreated and PRP treated cells were statistically significant (p<0.05).
41
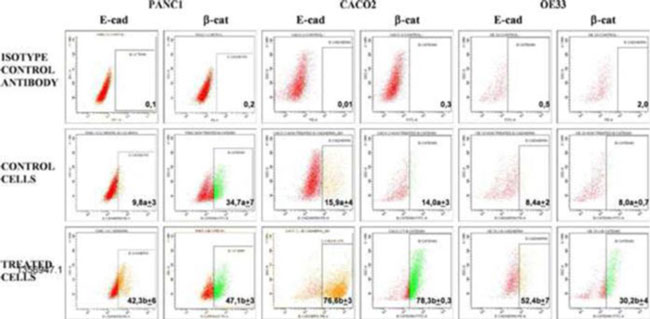
PRP has proven anti-tumor efficacy in Melanoma (B16-F10) Tumor Bearing Mice.
The anti-tumor activity of PRP was assessed in a B16-F10 melanoma model. The tumors were grafted under the skin in C57BL/6 female mice. The tumor-bearing mice were dosed with PRP twice daily, vehicle or control (doxorubicin (Adriblastine™) dosed once at 12 mg/kg i.v (i.e. approximately four-times the clinical dosage based on mg/kg conversion to a 60 kg human) for 28 consecutive days (n = 10 for each group). Treatment began 7 days post-implant.
During the course of the experiment, animals were sacrificed if any of the following occurred: signs of suffering (cachexia, weakening, difficulty moving or eating), compound toxicity (hunching, convulsions, diarrhea), tumor growing to 10% of body weight, tumor ulcerating and remaining open, position of tumor interfering with movement/feeding, 15% body weight loss for 3 consecutive days or 20% body weight loss for 1 day. The mice were sacrificed when the tumor volume reached a maximum volume of 2,000 mm.
Treatment with PRP was well tolerated following intra-peritoneal (i.p) injection into mice. Observations were specifically made to observe any drug-related toxicity (including hunching, convulsions, and diarrhea). There were no adverse events attributable to PRP, nor injection site reactions. Following 21 days treatment (28 days post-implantation), relative tumor volumes (defined as tumor volumes measured x number of days post treatment, divided by the tumor volume measured at day 0, post treatment) were significantly smaller in the i.p. and adriblastine groups compared to the untreated control.
This experiment shows our product, PRP has anti-tumor efficacy in mice, but without the severe, or even serious side effects normally associated with current treatment standards such as chemotherapy.
42
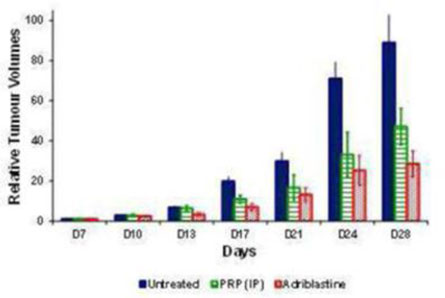
PRP-DCM
To date, we have been focused on developing a novel combination of anti-cancer agents working in combination with pro-enzymes which enhance PRP’s anti-cancer effects. The enhanced pro-enzymes-based formulations combine PRP with at least one of two types of identified compounds considered on the basis PRP’s mechanism of action to synergistically enhance the anti-cancer effects of PRP.
Our recent work has focused on maximizing the potential of PRP as a drug suitable for long-term maintenance by enhancing the effects of our current pro-enzyme formulation by screening additional active ingredients to enhance the anti-cancer activity of PRP.
Propanc’s scientists believe the additional ingredients identified in the course of this research to augment anti-cancer activity of PRP may also be suited as a stand-alone, adjunct therapy for standard treatment approaches, such as chemotherapy.
Anti-Cancer effects and mechanism of action
Cells obtain the energy they require from aerobic or anaerobic respiration (with, or without oxygen, respectively). It has been suggested that tumor cells rely on anaerobic respiration due to impairment of the mitochondria (an organelle found in most cells, in which the biochemical processes of respiration and energy production occur). We have identified compounds which have pronounced effects on the anaerobic cells within a tumor, which would complement PRP and standard treatment approaches:
| · | 2-deoxy-D-glucose, a metabolite which inhibits glucose metabolism in cancer cells, as reported in the British Journal of Cancer, 2002; | |
| · | Capsiate, a non-pungent component from sweet peppers, induces apoptosis by increasing the production of oxygen in cancer cells through forced up-regulation of cell mitochondria, published in the European Journal for Nutrition, 2003; | |
| · | Methyl-seleno-cysteine, which at low doses increases the oxidative stress on cancer cells by inhibiting a specific enzyme known to be up-regulated in tumor cells, published in Biochemical Pharmacology, 2008. |
Preclinical Development
In November 2010, we established collaborative research partnership with Dr. Paul Clayton, an expert in cancer prevention and nutrition and former advisor to the Committee on Safety of Medicines (UK), identifying specific anti-cancer agents in combination with one another, and with PRP, enhancing their ability to target cancerous cells with minimal side effects to healthy cells.
43
As a result of the work undertaken in collaboration with Dr. Paul Clayton, an international PCT application was filed late 2010, detailing enhanced pro-enzyme patent formulations and combination therapies comprising trypsinogen and chymotrypsinogen. Dr. Clayton was awarded a success fee in the form of shares of our common stock representing 1% of the shares then currently issued and outstanding in recognition of his contribution to this research. The patent application is jointly owned by us and the University of Bath, with an exclusive right and license to commercialize any joint intellectual property being held by Propanc (see under License Agreements and Intellectual Property for further details).
Effects on Cell Growth Inhibition Alone and In Combination
The interaction that occurs between agents can be described as synergistic, additive or antagonistic. The work we have conducted to enhance the anti-cancer effects of PRP focused on the positive therapeutic outcome of drug interactions, specifically synergism. The major benefits of additive and synergistic drug interactions are increased efficacy and significantly diminished toxic side effects. This can be achieved by reducing the dose of a drug that elicits damaging side effects, through a combination with another drug. Alternatively, a drug with insufficient efficacy could produce super-additive (synergistic) effects in a well-designed combination.
IC50 determination assays (the concentration of drug to cause 50% reduction in proliferation of cancer cells) were performed for 2-deoxyglucose, capsiate, methyl-seleno-cysteine and the mixture of these three components (i.e. DCM) in a human colorectal carcinoma cell line, HCT-15. IC50 values were obtained for 2-Deoxyglucose, capsiate and DCM. Methyl-seleno-cysteine treatment of the cells resulted in a maximum growth inhibition of 14.8% at the maximum tested concentration and therefore, an IC50 value was not obtained for this Test Article.
Following the IC50 determination assays, a scientific method was employed to study the interaction between 2-deoxy glucose, capsiate and methyl-seleno-cysteine. We found:
| · | capsiate, 2-deoxyglucose and DCM are inhibitors of the growth of the human colorectal carcinoma cell line HCT-15 in vitro; and | |
| · | methylselenocysteine and 2-Deoxyglucose synergise to inhibit the growth of the human colorectal carcinoma cell line HCT-15. |
We have also made several other similar observations with other compounds that act to inhibit the growth of the human colorectal carcinoma cell line HCT-15. Further work is needed to assess the optimal combination of ingredients before undertaking formal preclinical development of a potential new combination therapy in animals. We will determine a final combination to be developed as an adjunct therapy to PRP.
Preclinical development
The anti-angiogenic efficacy of PRP in combination with DCM was investigated using vivoPharm's AngioChamber™ assay. The AngioChamber™ assay utilises the normal physiological process of wound healing, to promote fibrous capsule formation around an implanted chamber in mice. The inclusion of Basic Fibroblast Growth Factor (bFGF) in the chamber supports the fibrouse capsule formation while inducing blood vessel development. Thus, this system is used to assess the efficacy of anti-angiogenic treatments by measuring fibrous capsule formation (wet weight of capsule at termination).
Fifty female FvB mice each received a subcutaneously implanted AngioChamber™, with or without bFGF. Ten mice were randomly selected and implanted with Chambers without bFGF. Forty mice which were implanted with Chambers containing bFGF were randomised by body weight into four treatment groups of 10 mice on Day 0 of the study. A reference compound, Sorafenib (60 mg/kg, per oral) was also introduced into the study.
In this Study both treatments resulted in significant inhibition of bFGF-induced angiogenesis compared with the Induction Control, as indicated by the capsule wet weights on the termination day of the study. Both the reference compound, Sorafenib, and the combination of PRP-DCM significantly reduced angiogenesis.
44
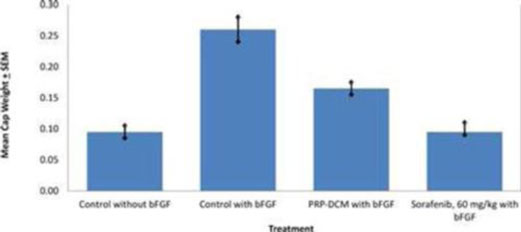
As is frequently seen in cancer research, animal cancer models using PRP and DCM in combination have in some instances shown very encouraging results, with less clear cut results in other animal models. We are working to understand which models are most appropriate, and how to further optimize the DCM formulation as a possible adjunct therapy for use, either in combination with PRP, or other standard treatment approaches.
The PRP Formulation
Oral pancreatic enzymes have been administered previously in a variety of circumstances, and are in current clinical use in conditions where the pancreas is unable to produce sufficient enzymes for the digestion of food. A number of oral pancreatic enzyme products are presently approved in the U.S for use in patients who do not produce enough pancreatic enzymes. Approved pancreatic enzyme products include Pancreaze™ from Johnson & Johnson, CREON® from Abbott Laboratories, and ULTRASE® from Axcan Pharma US.
PRP is a combination of two pro-enzymes trypsinogen and chymotrypsinogen, specifically formulated within a specific ratio designed to synergistically enhance their anti-cancer effects and in combination with other therapies identified based on the mechanism of action. Patent protection is currently being sought for PRP and other potential combinations, which forms part of the subject matter of International (PCT) Patent Application No. PCT/AU2010/001403 filed on 22 October 2010 in the name of Propanc Pty Ltd, our Australian operating subsidiary.
Oral enzymes have also been investigated previously for the treatment of cancer and, whilst generating encouraging results, their widespread use has been hampered by the very large quantities that have been considered necessary for effective treatment – 130 or more tablets per day. The high dose used with oral delivery is considered necessary due to the oral enzymes being broken down in the stomach and duodenum, the first part of the small intestine, and very little actually being absorbed into the general circulation. By administering a pro-enzyme by parenterally, and using a specific pro-enzyme formulation, the normal breakdown of the enzymes when taken orally is avoided and the drug can potentially be absorbed into the general circulation intact. It is also suggested that pro-enzymes are resistant to inactivation by numerous protein digesting enzymes, like serpins, which are circulating in the blood. Together with our scientific consultants, we believe that the development of a parenteral pro-enzyme formulation will lead to improved efficacy in the treatment of cancer compared with current oral enzyme preparations, and will substantially reduce the dose in comparison to that used previously for oral enzyme therapy for the treatment of cancer.
Our Research Programs
POP1
In order to maximize our proprietary knowledge on the use of pro-enzymes in the treatment of cancer, we are currently undertaking research to identify the mechanism at the molecular level by which PRP is acting to cause cancer cell death. A research program has been established with our collaborators at the University of Granada to investigate the changes in genetic and protein expression that occur in cancer cells as a consequence of being exposed to PRP. The objective of this work is to understand PRP on a molecular level changes in gene expression of the cancer cell post treatment. This will enable us to identify new, patentable drugs which we can develop such as synthetic recombinant proteins designed to improve the quality, safety and performance of pro-enzymes used in our current formulations.
45
Target Indications
The management of cancer differs widely, with a multitude of factors impacting on the choice of treatment strategy. Some of those factors include:
| · | the type of tumor, usually defined by the tissue in the body from which it originated; | |
| · | the extent to which it has spread beyond its original location; | |
| · | the availability of treatments, driven by multiple factors including cost, drugs approved, local availability of suitable facilities etc.; | |
| · | regional and geographic differences; | |
| · | whether the primary tumor is amenable to surgery, either as a potentially curative procedure, or as a palliative one; and | |
| · | the balance between potential risks and potential benefits from the various treatments, and probably most importantly, the patient’s wishes. |
For many patients with solid cancers, such as breast, colorectal, lung and pancreatic cancer, surgery is frequently the first treatment option, frequently followed by first line chemotherapy +/- radiotherapy. Whilst hopefully such procedures are curative, in many instances the tumor returns, and second line treatment strategies are chosen in an effort to achieve a degree of control of the tumor. In most instances, the benefit is temporary, and eventually the point is reached where the patient’s tumor either fails to adequately respond to treatment, or the treatment has unacceptable toxicity which severely limits its usefulness.
Should the proposed Phase I, II and III clinical trials confirm the efficacy of our product candidates, along with the excellent safety and tolerability profile suggested by pre-clinical studies conducted, to date, our product will have utility in a number of clinical situations including:
| · | in the early stage management of solid tumors, most likely as part of a multi-pronged treatment strategy in combination with existing therapeutic interventions; | |
| · | as a product that can be administered long term for patients following standard treatment approaches, such as surgery, or chemotherapy, in order to prevent or delay recurrence. | |
| · | as a preventative measure for patients at risk of developing cancer based on genetic screening. |
In the near term, we plan to target patients with solid tumors, most likely colorectal and pancreatic tumors, for whom other treatment options have been exhausted. This is a common approach by which most new drugs for cancer are initially tested. Once efficacy and safety has been demonstrated in this patient population, exploration of the potential utility of the drug in earlier stage disease can be undertaken, together with investigation of the drug’s utility in other types of cancer.
Development Strategy
Our goal is to undertake early stage non-clinical and clinical development of our drug products through to a significant value inflexion point, where the commercial attractiveness of a drug in development, together with a greater likelihood of achieving market authorization, may attract potential interest from licensees seeking to acquire new products. Such value inflexion points in the context of cancer drugs are typically at the point where formal, controlled clinical trials have demonstrated either ‘efficacy’ or ‘proof of concept’ – typically meaning that there is controlled clinical trial evidence that the drug is effective in the proposed target patient population, has an acceptable safety profile, and is suitable for further development. From a ‘big picture’ perspective, it is our intention to progress the development of its technology through to completion of Phase I clinical trials and then to seek a licensee for further development beyond that point.
46
As part of that commercial strategy, we will:
| · | continue research and development to build our existing intellectual property portfolio, and to seek new, patentable discoveries; | |
| · | seek to ensure all product development is undertaken in a manner that makes its products approvable in the major pharmaceutical markets, including the U.S., Europe, the UK and Japan; | |
| · | aggressively pursue the protection of our technology through all means possible, including patents in all major jurisdictions, and potentially trade secrets; | |
| · | make strategic acquisitions to acquire new companies that have products or services that complement our future goals. |
Development Plan and Milestones
PRP
We plan to progress PRP down a conventional non-clinical and early stage clinical development pathway either in Central, or Eastern Europe for:
| · | the manufacture of PRP for non-clinical development; | |
| · | non-clinical safety toxicology studies; | |
| · | regulatory approval to conduct a Phase I study in the relevant country, and submit it to the applicable regulatory authority for approval; and | |
| · | Phase I single escalating and multiple escalating dose studies to investigate the safety, tolerability, and pharmacokinetics of PRP injection in healthy male subjects. |
We anticipate reaching the Phase IIa proof of concept milestone in approximately three years, subject to regulatory approval in Europe and the US, and the results from our research and development and licensing activities.
Our overheads are likely to increase from its current level as our lead product candidate, PRP progresses down the development pathway. This increase will be driven by the need to increase our internal resources in order to effectively manage our research and development activities.
We are currently raising sufficient capital to complete Phase I clinical trials over the next twenty-four months, although additional capital may be sought after twelve months to support expansion of research and development activities and our overheads (assuming planned expansion of internal resources are approved internally and completed accordingly).
Anticipated timelines
| 2015 | 2016 | 2017 | |||||||||||||||
| Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | ||||||||||
| Animal efficacy models on PRP completed | X | ||||||||||||||||
| Non-clinical development | X | X | X | ||||||||||||||
| Conduct scientific advice meetings with regulatory agencies | X | ||||||||||||||||
| Manufacturing, production of drug substance and product for preclinical and clinical trials | X | X | X | ||||||||||||||
| Non-clinical development | X | X | X | ||||||||||||||
| Obtain regulatory approval | X | ||||||||||||||||
| Phase I | X | X | X | ||||||||||||||
47
For the period from October to November 2015, we completed animal efficacy models on PRP. Preparation is underway for scientific advice meetings with regulatory agencies to discuss our non-clinical and clinical development pathway for PRP, early 2016.
It is anticipated for the period from March to December 2016, we intend to complete the manufacturing, production of drug substance and product for preclinical and clinical trials, as well as undertaking formal toxicology studies. We anticipate the cost to be $600,000 and $650,000, respectively.
For the period from January to September 2017, we intend to initiate and then complete a Phase I study in advanced cancer patients with solid tumors and the anticipated costs will be $900,000 approximately.
Non Clinical Development
Cell line studies have been performed optimizing the ratio of the two proenzymes in our product, PRP. These studies demonstrate synergistic activity over the individual components. Maximum tolerated and feasible dose and pilot animal efficacy studies were undertaken showing no adverse clinical signs at higher doses and substantial tumor growth inhibition in pancreatic and ovarian cancers. Additional pharmacokinetic analysis is underway, and toxicology studies will be completed in the near future.
Bio-analytical assays for PRP will be developed prior to commencing the dose-range finding studies. In addition, the potential for E-cadherin to be used as a biomarker for PRP activity will be explored.
We are planning to develop PRP as a parenteral formulation. Consequently, dose selection for GLP safety toxicology studies will be determined. Twenty – eight day GLP safety studies may also be necessary for PRP.
Clinical Development
It is proposed to perform the first-in-human studies in healthy male subjects as opposed to advanced cancer patients, given the favorable safety profile of PRP which appears less toxic than standard treatments. The studies will assess the safety and tolerability of PRP given either as single or repeated once daily subcutaneous injections compared to placebo under the same conditions. They will be mono-center, double-blind, randomized, safety, tolerability and pharmacokinetic studies. It is planned to escalate the dose until a maximum tolerated single dose, and then repeated dose, of PRP is reached. Eight male subjects, followed by twelve male subjects, will be evaluated for the single and repeated dose studies, respectively.
After the safety and tolerability studies are completed, a multi-center, open Phase II study evaluating the efficacy and safety of PRP administered to patients with locally advanced or metastatic pancreatic adenocarcinoma will be conducted. Initially, twenty – three patients will be recruited for the first stage. If two or more responders are identified, thirty – three additional patients will be recruited. The primary objective will be to evaluate the overall survival in patients with advanced pancreatic adenocarcinoma having received once daily subcutaneous injections of PRP.
POP1
As outlined previously, a research program has been established with our collaborators at the University of Granada to investigate the changes in genetic and protein expression that occur in cancer cells as a consequence of being exposed to our pro-enzyme formulation. The objective of this work is to understand at the molecular level the targets of our pro-enzyme formulation, thereby providing the opportunity for new, patentable drugs which can be developed further. We plan to commence a targeted drug discovery program utilizing the identified molecular target to search for novel anticancer agents.
Financial Objectives
Multiple factors, many of which are outside of Propanc’s control, can impact on the ability of Propanc to achieve its target objectives within the planned time and budgetary constraints. Subject to these caveats, it is Propanc’s objective to achieve the following R&D milestones within the proposed budget:
48
| · | PRP completed Phase I clinical trial. | |
| · | Development candidate identified from the POP1 program. |
Corporate Strategy
We operate as a ‘virtual’ company contracting services, skills and expertise as required to achieve our scientific and corporate objectives. As the business grows and gains more personnel, outsourcing will continue to be the preferred model, where fixed and variable costs are carefully managed on a project by project basis. This means our research and development activities will be carried out by third parties. So far, we have engaged our research partners from the Universities of Bath and Granada. Additional third parties with specific expertise in research, compound screening and manufacturing (including raw material suppliers) will be contracted as required. Initial discussions have been held with several third parties and will be contracted as we progress into the next stages of the development process.
Our initial focus will be to organize, coordinate and finance the various parts of the drug development pipeline. New personnel will be carefully introduced into the company over a period of time as the company’s research and development activities expand. They will have specific expertise in product development, manufacture & formulation, regulatory affairs, toxicology, clinical operations and business development (including intellectual property management, licensing and other corporate activities).
In the first instance, additional clinical management and development expertise is likely to be required for our lead product therefore we anticipate an increase in employees in order to effectively manage our contractors as the project progress down the development pathway.
This outsourcing strategy is common in the biotechnology sector, and is an efficient way to obtain access to the necessary skills required to progress a project, in particular as the required skills change as the project progresses from discovery, through manufacturing and non-clinical development, and into clinical trials. We anticipate continuing to utilize this model, thereby retaining the flexibility to contract in the appropriate resource as and when required.
We intend to seek and identify potential licensing partners for our product candidates as they progress through the various development stages, reaching certain milestones and value inflection points. If a suitable licensee is identified, a potential licensing deal could consist of payments for certain milestones, plus royalties from future sales if the product is able to receive approval the relevant regulatory authorities where future product sales are targeted. We intend to seek and identify potential licensees based on the initial efficacy data from Phase I clinical trials within the next 18 to 24 months.
As part of our overall expansion strategy, we are investigating potential intellectual property acquisition opportunities to expand our product portfolio. Whilst the company’s initial focus is on the development of PRP as the lead product candidate, potential product candidates may also be considered for future preclinical and clinical development. These potential opportunities have arisen from other research and development organizations, which either own existing intellectual property, or currently developing new intellectual property, which may be of interest to us. These potential opportunities are potentially new cancer treatments which are potentially less toxic than existing treatment approaches and are able to fill an existing gap in the treatment process, such as a systemic de-bulking method which could reduce the size and threat of metastases to a more manageable level for late stage cancer patients. We believe these potential treatment approaches will be complementary to existing treatment regimens and our existing product candidate, PRP. No formal approaches have been made at this stage and it is unknown whether we will engage in this discussion in the near future. However, we remain hopeful that as PRP progresses further down the development pathway, future opportunities may arise to utilize the expertise of our management and scientific personnel for future prospective research and development projects.
49
Current Operations
We are at a pre-revenue stage. We do not know when, if ever, we will be able to commercialize our products and begin generating revenue, we are focusing our efforts on organizing, coordinating and financing the various aspects of the drug research and development program outlined earlier in this document. In order to commercialize our products, we must complete preclinical development, and Phase I, II and III clinical trials in Europe, the U.S., Australia, or elsewhere, and satisfy the applicable regulatory authority that PRP is safe and effective. We estimate that this will take approximately seven years. As described previously, when we have advanced our development projects sufficiently down the development pathway to achieve a major increase in value, such as obtaining interim efficacy data from Phase I clinical trials, we will seek a suitable licensing partner to complete the remaining development activities, obtain regulatory approval, and market the product.
Current Therapies/Drugs Available
We are developing a therapeutic solution for the treatment of patients with advanced stages of cancer targeting solid tumors, which is cancer that originates in organs or tissues other than bone marrow or the lymph system. Common cancer types classified as solid tumors include lung, colorectal, ovarian cancer, pancreatic cancer and liver cancers. In each of these indications, there is a large market opportunity to capitalize on the limitations of current therapies.
Current therapeutic options for the treatment of cancer offer, at most, a few months of extra life or tumor stabilization. Some experts believe that drugs that kill most tumor cells do not affect cancer stem cells which can regenerate the tumor (e.g. chemotherapy). Studies are revealing the genetic changes in cells that cause cancer and spur its growth this research is providing scientific researchers with dozens of potential “targets” for drugs. Tumor cells, however, can develop resistance to drugs.
Limitations of Current Therapies
PRP was developed because of the limitation of current cancer therapies. While surgery is often safe and effective for early stage cancer, many standard therapies for late stage cancer urgently need improvement; with current treatments generally providing modest benefits, and frequently causing significant adverse effects. Our focus is to provide oncologists and their patients with therapies for metastatic cancer which are more effective than current therapies, and which have a substantially reduced side effect profile.
While progress has been made within the oncology sector in developing new treatments, the overall cancer death rate has only improved 7% over the last 30 years. Most of these new treatments have some limitations, such as:
| · | significant toxic effects; | |
| · | expense; and | |
| · | limited survival benefits. |
We believe that our treatment will provide a competitive advantage over the following treatments:
| · | Chemotherapeutics: Side effects from chemotherapy can include pain, diarrhea, constipation, mouth sores, hair loss, nausea and vomiting, as well as blood-related side effects, which may include a low number of infection fighting white blood cell count (neutropenia), low red blood cell count (anemia), and low platelet count (thrombocytopenia). Our goal is to demonstrate that our treatment will be more effective than chemotherapeutic and hormonal therapies with fewer side effects. |
| · | Targeted therapies: Most common type is multi-targeted kinase inhibitors (molecules which inhibit a specific class of enzymes called kinases). Common side effects include fatigue, rash, hand–foot reaction, diarrhea, hypertension and dyspnoea (shortness of breath). Furthermore, tyrosine kinases inhibited by these drugs appear to develop resistance to inhibitors. Whilst the clinical findings with PRP are early and subject to confirmation in future clinical trials, no evidence has yet been observed of the development of resistance by the cancer to PRP. |
| · | Monoclonal antibodies: Development of monoclonal antibodies is often difficult due to safety concerns. Side effects which are most common include skin and gastro-intestinal toxicities. For example, several serious side effects from Avastin, an anti-angiogenic cancer drug, include gastrointestinal perforation and dehiscence (e.g. rupture of the bowel), severe hypertension (often requiring emergency treatment) and nephrotic syndrome (protein leakage into the urine). Antibody therapy can be applied to various cancer types in some cases, but can also be limited to certain genetic sub populations in many instances. |
50
| · | Immunotherapy: There is a long history of attempts to develop therapeutic cancer vaccines to stimulate the body’s own immune system to attack cancer cells. These products, whilst they generally do not have the poor safety profile of standard therapeutic approaches, have rarely been particularly effective. Whilst there are a number of therapeutic cancer vaccines currently in development, most are in the early stages of clinical development. To date, only one therapeutic cancer vaccine has been approved by the US Food and Drug Administration. |
Market Opportunity
Total global oncology drug sales reached $91 billion in 2013 and are growing at 5% annually. In particular, targeted therapies have significantly increased their share from 11% a decade ago to 46% last year. Biological products, which are products often made from natural resources, such as human, animal and microorganisms, represent nearly half of the oncology market. More recently, new drug launches have concentrated on small molecules, including kinase inhibitors. However, these new drugs cost more because they are meant for smaller patient populations.
Our cancer treatment is intended to be positioned among the five types of cancer drug classes currently contributing to the significant growth in the oncology market. The five main drug classes are chemotherapeutics, hormonals, immunotherapy and vaccines, targeted therapies and monoclonal antibodies.
Demand for new cancer products can largely be attributed to a combination of a rapidly aging population in western countries and changing environmental factors, which together are resulting in rising cancer incidence rates. According to the World Health Organization, all cancers (excluding non-melanoma skin cancer) are expected to increase from 8.2 million annual deaths in 2012 to over 10 million annual deaths by 2020, exceeding 13 million annual deaths by 2030. As such, global demand for new cancer treatments which are effective, safe and easy to administer is rapidly increasing. Our treatment will potentially target many aggressive tumor types for which little or few treatment options exist.
We plan to target patients with solid tumors, most likely colorectal and pancreatic tumors, for whom other treatment options have been exhausted. Globally these cancers resulted in over 694,000 deaths per year in 2012. With such a high mortality rate, a substantial unmet medical need exists for new treatments.
For example, current standard treatment for colorectal cancer consists of cytotoxics, which are associated with high levels of toxicity. Despite the relatively recent approval of Erbitux™ and Avastin™, both of which are monoclonal antibodies, for the treatment of colorectal cancer, significant treatment-related adverse effects continue to be problematic for patients with colorectal cancer. The need exists for tolerable agents that will improve quality of life for patients as well as offering a potential cure (Datamonitor, 2004).
For pancreatic cancer, there is a lack of effective therapies on the market for pancreatic cancer and any newly approved agents with some efficacy are likely to see significant uptake once commercialized (Datamonitor, 2004). Targeted therapies may fulfill this need, although further intensive research and development is necessary.
Once the efficacy and safety of PRP has been demonstrated in late stage patient populations, we plan to undertake exploration of the utility of the drug in earlier stage disease, together with investigation of the drug’s utility in other types of cancer.
Anticipated Market Potential
It is difficult to estimate the size of the market opportunity for this specific type of product as a clinically proven, pro-enzyme formulated suppository marketed to oncologists across global territories for specific cancer indications, to the best of management’s knowledge, has not been previously available.
However, the markets for potential market for colorectal and pancreatic cancer may be characterized as follows:
51
| · | Colorectal cancer: In 2011, according to available information online, the global colorectal cancer therapeutics market was worth $8.3 billion. The market is expected to decrease marginally to $7.8 billion by 2021 because of generic competition for a key cytotoxic agent, oxaliplatin, as well as the entry of biosimilar competitors for key targeted biological agents. Therefore, demand for new and innovative treatment approaches will be significant to support future growth and continue to improve treatment standards. |
| · | Pancreatic cancer: The world market for pancreatic cancer drugs is projected to grow to $1.63 billion by the year 2017. This rapid market expansion is due to launch of Celgene’s Abraxane in the US and Europe in 2013 and 2014. Abraxane will represent nearly 60% of the cancer therapeutics market by the end of 2017. |
Based on the current situation for these two markets, we believe there is an attractive opportunity in both the colorectal and pancreatic cancer market sectors for the introduction of a clinically proven product which can achieve new benefits for patients in terms of survival and quality of life. The current concentration of products suggests oncologists may be willing to try newly approved products, particularly if they can exhibit a favorable safety profile, although substantive R&D activities will be necessary to both obtain regulatory approval, and to generate the clinical safety and efficacy data needed to convince clinicians to use a new product.
License Agreements
We previously sponsored a collaborative research project at Bath University to investigate the cellular and molecular mechanisms underlying the potential clinical application of our proprietary pro-enzyme formulation.
As a result of this undertaking, we entered into a Commercialization Agreement with Bath University, dated 12 November 2009, where, initially, Propanc, held an exclusive license with the University of Bath (UK), where we, and the University, co-own the intellectual property relating our pro-enzyme formulations. The Commercialization Agreement provides for Bath to assign the Patents to Propanc in certain specified circumstances, such as successful completion of a Phase I clinical trial and commencement of a Phase IIa (Proof of Concept) clinical trial.
On June 14 2012, Propanc and Bath University agreed to an earlier assignment of the patents pursuant to an Assignment and Amendment Deed, on the proviso that Bath retains certain rights arising from the Commercialization Agreement, as follows:
| · | Bath reserves for itself (and its employees and students and permitted academic sub-licensees regarding Research Use) the non-exclusive, irrevocable, worldwide, royalty free right to use the Patents for Research Use. |
| · | The publication rights of Bath specified in the contract relating to the Original Research made between the Parties with an effective date of 18th July 2008 shall continue in force. |
| · | Propanc shall pay to the University of Bath a royalty being two (2) per cent of any and all net revenues. |
| · | Propanc shall use all reasonable endeavors to develop and commercially exploit the Patents for the mutual benefit of Bath and Propanc to the maximum extent throughout the Territory in the Field and in each Additional Field and to obtain, maintain and/or renew any licenses or authorizations which are necessary to enable such development and commercial exploitation. Without prejudice to the generality of the foregoing, Propanc shall comply with all relevant regulatory requirements in respect of its sponsoring and/or performing clinical trials in man involving the administration of a product or materials within a claim of the Patents. |
| · | Propanc shall take out with a reputable insurance company and maintain liability insurance cover prior to the first human trials. |
We have been working together with the University of Bath to patent and commercialize these discoveries, while continuing to elucidate the properties of pro-enzymes with the long-term aim of screening new compounds for development. At present, we are engaged in discussions with several technology companies who are progressing new developments in the oncology field as potential additions to our product line. Initially targeting the oncology sector, our focus is to identify and develop novel treatments which are highly effective targeted therapies, with few side effects as a result of toxicity to healthy cells.
52
Intellectual Property
We have filed an international patent application directed to enhance pro-enzyme formulations and combination therapies comprising trypsinogen and chymotrypsin, and/or a number of other specific anti-cancer agents. The international patent application has been based on previous provisional patent applications filed by us capturing ongoing research and development in this area.
The international patent application was filed on October 22, 2010, which claims priority for Australian provisional patent application nos. 2009905147 (filed October 22, 2010) and 2010902655 (filed June 17, 2010).
The details of such patent are as follows:
| · | Title: A Pharmaceutical Composition For Treating Cancer Comprising Trypsinogen And/Or Chymotrypsinogen And An Active Agent Selected From A Selenium Compound, A Vanilloid Compound, And A Cytoplasmic Glycolysis Reduction Agent. |
| · | Date filed: 22nd October 2010. |
| · | Jurisdiction: The Patent Cooperation Treaty or PCT is an international agreement for filing patent applications having effect in up to 117 countries. Under the PCT, an inventor can file a single international patent application in one language with one patent office in order to simultaneously seek protection for an invention in up to 117 countries. |
We completed the 30-month national phase filing deadline for this international PCT application and commenced entering the national phase in individual countries and regions, including United States, Canada, Japan, Brazil, China, Mexico, Hong Kong, Singapore, Israel, Chile, Peru, Malaysia, Vietnam, Indonesia, Europe, Russia, India, and South Korea. The patent is now granted in South Africa, Australia, and New Zealand. Further, provisional patents are also currently being prepared and expected to be filed to capture and protect additional patentable subject matter that is identified, namely further enhanced formulations, combination treatments, use of recombinant products, modes of action and molecular targets.
More recently, an Australian provisional patent application #2015904678 was filed on November 12, 2015, relating to proenzyme compositions.
On January 29, 2016, a Spanish provisional patent application #201630112 was filed citing a method to treat cancer by eradicating cancer stem cells whilst sparing normal stem cells.
Our intellectual property portfolio also includes an extensive amount of confidential information, know-how and expertise in relation to the development and formulation of our pro-enzyme based combination therapies.
The basis of our intellectual property protection will be built around the following elements:
| · | Method of use: Understanding the mechanism of action of the PRP pro-enzyme formulations, enabling the identification of new molecular targets, potential new therapeutic compounds and identification of new formulations that are adapted to enhance activity. |
| · | Formulation: We have developed an enhanced formulation containing the pro-enzyme trypsinogen in combination with at least one of two types of identified compounds considered effective for providing synergistic enhancement of the pro-enzyme based formulations. A patentability assessment, based on an international prior art search, has indicated that strong potential exists for successfully obtaining patent claims covering the formulation. |
| · | Composition of Matter: Synthetic recombinant proteins designed to improve the quality, safety and performance of pro-enzymes used in the proposed formulations form part of the research and development program. |
53
Regulatory Issues
United States
Government oversight of the pharmaceutical industry is usually classified into pre-approval and post-approval categories. Most of the therapeutically significant innovative products marketed today are the subject of New Drug Applications (“NDA”). Preapproval activities, based on these detailed applications, are used to assure the product is safe and effective before marketing. In the United States, The Center for Drug Evaluation and Research, or CDER, is the Food and Drug Administration (the “FDA”) organization responsible for over-the-counter and prescription drugs, including most biological therapeutics, and generic drugs.
Before approval, the FDA may inspect and audit the development facilities, planned production facilities, clinical trials, institutional review boards, and laboratory facilities in which the product was tested in animals. After the product is approved and marketed, the FDA uses different mechanisms for assuring that firms adhere to the terms and conditions of approval described in the application and that the product is manufactured in a consistent and controlled manner. This is done by periodic unannounced inspections of production and quality control facilities by FDA’s field investigators and analysts.
Federal Food, Drug and Cosmetic Act and Public Health Service Act
Prescription drug and biologic products are subject to extensive pre- and post-market regulation by the FDA, including regulations that govern the testing, manufacturing, safety, efficacy, labelling, storage, record keeping, advertising and promotion of such products under the Federal Food, Drug and Cosmetic Act, the Public Health Service Act, and their implementing regulations. The process of obtaining FDA approval and achieving and maintaining compliance with applicable laws and regulations requires the expenditure of substantial time and financial resources. Failure to comply with applicable FDA or other requirements may result in refusal to approve pending applications, a clinical hold, warning letters, civil or criminal penalties, recall or seizure of products, partial or total suspension of production or withdrawal of the product from the market. FDA approval is required before any new drug or biologic, including a new use of a previously approved drug, can be marketed in the United States. All applications for FDA approval must contain, among other things, information relating to safety and efficacy, stability, manufacturing, processing, packaging, labelling and quality control.
New Drug Applications (NDAs)
The FDA’s NDA approval process generally involves:
| · | Completion of preclinical laboratory and animal testing in compliance with the FDA’s good laboratory practice, or GLP, regulations; |
| · | Submission to the FDA of an investigational new drug (“IND”) application for human clinical testing, which must become effective before human clinical trials may begin in the United States; |
| · | Performance of adequate and well-controlled human clinical trials to establish the safety, purity and potency of the proposed product for each intended use; |
| · | Satisfactory completion of an FDA pre-approval inspection of the facility or facilities at which the product is manufactured to assess compliance with the FDA’s “current good manufacturing practice” (“CGMP”) regulations; and |
| · | Submission to and approval by the FDA of a NDA. |
The preclinical and clinical testing and approval process requires substantial time, effort and financial resources, and Propanc cannot guarantee that any approvals for our product candidates will be granted on a timely basis, if at all. Preclinical tests include laboratory evaluation of toxicity and immunogenicity in animals. The results of preclinical tests, together with manufacturing information and analytical data, are submitted as part of an IND application to the FDA. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA raises concerns or questions about the conduct of the clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before clinical trials can begin. Our submission of an IND may not result in FDA authorization to commence clinical trials. A separate submission to an existing IND must also be made for each successive clinical trial conducted during product development. Further, an independent institutional review board (“IRB”), covering each medical centre proposing to conduct clinical trials must review and approve the plan for any clinical trial before it commences at that center and it must monitor the study until completed. The FDA, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk. Clinical testing also must satisfy extensive “good clinical practice” (“GCP”) regulations, which include requirements that all research subjects provide informed consent and that all clinical studies be conducted under the supervision of one or more qualified investigators.
54
For purposes of an NDA submission and approval, human clinical trials are typically conducted in the following sequential phases, which may overlap:
| · | Phase I: Trials are initially conducted in a limited population to test the product candidate for safety and dose tolerance. |
| · | Phase II: Trials are generally conducted in a limited patient population to identify possible adverse effects and safety risks, to determine the initial efficacy of the product for specific targeted indications and to determine dose tolerance and optimal dosage. Multiple Phase II clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more extensive Phase III clinical trials. |
| · | Phase III: These are commonly referred to as pivotal studies. When Phase II evaluations demonstrate that a dose range of the product is effective and has an acceptable safety profile, Phase III clinical trials are undertaken in large patient populations to further evaluate dosage, to provide substantial evidence of clinical efficacy and to further test for safety in an expanded and diverse patient population at multiple, geographically-dispersed clinical trial sites. Generally, replicate evidence of safety and effectiveness needs to be demonstrated in two adequate and well-controlled Phase III clinical trials of a product candidate for a specific indication. These studies are intended to establish the overall risk/benefit ratio of the product and provide adequate basis for product labelling. |
| · | Phase IV: In some cases, the FDA may condition approval of a NDA on the sponsor’s agreement to conduct additional clinical trials to further assess the product’s safety, purity and potency after NDA approval. Such post-approval trials are typically referred to as Phase IV clinical trials. |
Progress reports detailing the results of the clinical studies must be submitted at least annually to the FDA and safety reports must be submitted to the FDA and the investigators for serious and unexpected adverse events. Concurrent with clinical studies, sponsors usually complete additional animal studies and must also develop additional information about the product and finalize a process for manufacturing the product in commercial quantities in accordance with CGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things; the manufacturer must develop methods for testing the identity, strength, quality and purity of the final product. Moreover, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
The results of product development, preclinical studies and clinical trials, along with the aforementioned manufacturing information, are submitted to the FDA as part of a NDA. NDA’s must also contain extensive manufacturing information. Under the Prescription Drug User Fee Act, or PDUFA, the FDA agrees to specific goals for NDA review time through a two-tiered classification system, Standard Review and Priority Review. Standard Review is applied to products that offer at most, only minor improvement over existing marketed therapies. Standard Review NDAs have a goal of being completed within a ten-month timeframe, although a review can take a significantly longer amount of time. A Priority Review designation is given to products that offer major advances in treatment, or provide a treatment where no adequate therapy exists. A Priority Review means that the time it takes the FDA to review a NDA is six months. It is likely that our product candidates will be granted Standard Reviews. The review process is often significantly extended by FDA requests for additional information or clarification. The FDA may refer the application to an advisory committee for review, evaluation and recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations.
55
The FDA may deny approval of a NDA if the applicable regulatory criteria are not satisfied, or it may require additional clinical data or additional pivotal Phase III clinical trials. Even if such data are submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Data from clinical trials are not always conclusive and the FDA may interpret data differently than Propanc do. Once issued, product approval may be withdrawn by the FDA if ongoing regulatory requirements are not met or if safety problems occur after the product reaches the market. In addition, the FDA may require testing, including Phase IV clinical trials, Risk Evaluation and Mitigation Strategies, or REMS, and surveillance programs to monitor the effect of approved products that have been commercialized, and the FDA has the power to prevent or limit further marketing of a product based on the results of these post-marketing programs. Products may be marketed only for the approved indications and in accordance with the provisions of the approved label. Further, if there are any modifications to the drug, including changes in indications, labelling or manufacturing processes or facilities, approval of a new or supplemental NDA may be required, which may involve conducting additional preclinical studies and clinical trials.
Other U.S. Regulatory Requirements
After approval, products are subject to extensive continuing regulation by the FDA, which include company obligations to manufacture products in accordance with GMP, maintain and provide to the FDA updated safety and efficacy information, report adverse experiences with the product, keep certain records and submit periodic reports, obtain FDA approval of certain manufacturing or labelling changes, and comply with FDA promotion and advertising requirements and restrictions. Failure to meet these obligations can result in various adverse consequences, both voluntary and FDA-imposed, including product recalls, withdrawal of approval, restrictions on marketing, and the imposition of civil fines and criminal penalties against the NDA holder. In addition, later discovery of previously unknown safety or efficacy issues may result in restrictions on the product, manufacturer or NDA holder.
Propanc, and any manufacturers of our products, are required to comply with applicable FDA manufacturing requirements contained in the FDA’s GMP regulations. GMP regulations require among other things, quality control and quality assurance as well as the corresponding maintenance of records and documentation. The manufacturing facilities for our products must meet GMP requirements to the satisfaction of the FDA pursuant to a pre-approval inspection before Propanc can use them to manufacture our products. Propanc, and any third-party manufacturers, are also subject to periodic inspections of facilities by the FDA and other authorities, including procedures and operations used in the testing and manufacture of our products to assess our compliance with applicable regulations.
With respect to post-market product advertising and promotion, the FDA imposes a number of complex regulations on entities that advertise and promote pharmaceuticals, which include, among others, standards for direct-to-consumer advertising, promoting products for uses or in patient populations that are not described in the product’s approved labelling (known as “off-label use”), industry-sponsored scientific and educational activities, and promotional activities involving the Internet. Failure to comply with FDA requirements can have negative consequences, including adverse publicity, enforcement letters from the FDA, mandated corrective advertising or communications with doctors, and civil or criminal penalties. Although physicians may prescribe legally available drugs for off-label uses, manufacturers may not market or promote such off-label uses.
Changes to some of the conditions established in an approved application, including changes in indications, labelling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. A NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs.
Adverse event reporting and submission of periodic reports is required following FDA approval of a NDA. The FDA also may require post-marketing testing, known as Phase IV testing, risk mitigation strategies and surveillance to monitor the effects of an approved product or place conditions on an approval that could restrict the distribution or use of the product.
56
European Union
In addition to regulations in the United States, Propanc will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. Whether or not Propanc obtains FDA approval for a product, Propanc must obtain approval of a product by the comparable regulatory authorities of foreign countries before Propanc can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials product licensing, pricing and reimbursement vary greatly from country to country.
Under European Union regulatory systems, Propanc must submit and obtain authorization for a clinical trial application in each member state in which Propanc intend to conduct a clinical trial. After Propanc have completed clinical trials, Propanc must obtain marketing authorization before Propanc can market its product. Propanc must submit applications for marketing authorizations for oncology products under a centralized procedure. The centralized procedure provides for the grant of a single marketing authorization that is valid for all European Union member states. The European Medicines Agency (the “EMA”) is the agency responsible for the scientific evaluation of medicines that are to be assessed via the centralized procedure.
Australia
In Australia, the relevant regulatory body responsible for the pharmaceutical industry is the Therapeutics Goods Administration (the “TGA”). Prescription medicines are regulated under the Therapeutic Goods Act 1989. Under the Therapeutic Goods Act, the Therapeutic Goods Administration evaluates new products for quality, safety and efficacy before being approved for market authorization, according to similar standards employed by the FDA and EMA in the United States and European Union, respectively. However, receiving market authorization in one or two regions does not guarantee approval in another.
Third-Party Payor Coverage and Reimbursement
Although none of our product candidates have been commercialized for any indication, if they are approved for marketing, commercial success of our product candidates will depend, in part, upon the availability of coverage and reimbursement from third-party payors at the federal, state and private levels.
Other Regulations
We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances. We may incur significant costs to comply with such laws and regulations now or in the future.
Competition
The biotechnology and pharmaceutical industries are characterized by continuing technological advancement and significant competition. While we believe that our technology platforms, product candidates, know-how, experience and scientific resources provide us with competitive advantages, we face competition from major pharmaceutical and biotechnology companies, academic institutions, governmental agencies and public and private research institutions, among others. Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future. Key product features that would affect our ability to effectively compete with other therapeutics include the efficacy, safety and convenience of our products. The level of generic competition and the availability of reimbursement from government and other third-party payers will also significantly impact the pricing and competitiveness of our products. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market.
Many of the companies against which we may compete have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
57
Employees
As of March 22, 2016, we currently have one (1) full time employee and one (1) part-time employee. Over time, we may be required to hire employees or engage independent contractors in order to execute various projects necessary to grow and develop the business. These decisions will be made by our officers and directors, if and when appropriate.
Our principal executive office is located at Level 2, 555 Riversdale Road, Camberwell, VIC, 3124 Australia. The lease costs approximately $2,200 AUD per month and expires on three months’ notice by either Propanc or the leasing company.
From time to time, we may be involved in litigation in the ordinary course of business.
The Company has been in an arbitration proceeding brought by Typenex Co-Investment, LLC, a Utah limited liability company (“Typenex”) relating to a loan made by Typenex to the Company in June and July of 2015. In mid-February 2016, Typenex was able to fully convert its debt to the 31,411,463 shares in Propanc. Typenex currently claims late fees and interest in the and the Company has has submitted counterclaims against Typenex. An arbitration hearing has been set for the summer of 2016. The Company does not believe the result of this litigation matter will have a material adverse effect on our financial conditions or results of operations.
The Company negotiated a settlement with JMJ Financial Inc., a Florida corporation (“JMJ”), and on December 10, 2015, the Company repaid cash of $90,000 as payment in full of the promissory note and for full satisfaction and accord of all JMJ’s claims.
Other than above, to the best of our knowledge, there are no other material pending legal proceedings to which we are a party or of which any of our property is the subject.
MARKET FOR COMMON EQUITY AND RELATED SHAREHOLDER MATTERS
Price Range of Common Stock
Our Common Stock is quoted under the ticker symbol “PPCH” on the OTCQB operated by OTC Markets Group, Inc. Only a limited market exists for our securities. There is no assurance that a regular trading market will develop, or if developed, that it will be sustained. Therefore, a shareholder may be unable to resell his securities in our company.
The following table sets forth the range of high and low bid quotations for our common stock for each of the periods indicated as reported by the OTCQB. These quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
| High Bid* ($) |
Low Bid* ($) |
|||||||
| Second quarter ended December 31, 2015 | $ | 0.05 | 0.26 | |||||
| First quarter ended September 30, 2015 | $ | 0.09 | 0.23 | |||||
| Fiscal Year Ended June 30, 2015 | ||||||||
| Fourth quarter ended June 30, 2015 | $ | 0.1338 | 0.014 | |||||
| Third quarter ended March 31, 2015 | $ | 0.04 | 0.001 | |||||
| Second quarter ended December 31, 2014 | $ | 0.0189 | 0.0012 | |||||
| First quarter ended September 30, 2014 | $ | 0.11 | 0.015 | |||||
| Fiscal Year Ended June 30, 2014 | ||||||||
| Fourth quarter ended June 30, 2014 | $ | 0.465 | 0.08 | |||||
| Third quarter ended March 31, 2014 | $ | 0.10 | 0.10 | |||||
| Second quarter ended December 31, 2013 | $ | 0.20 | 0.10 | |||||
| First quarter ended September 30, 2013 | $ | 0.20 | 0.20 | |||||
* The quotations of the closing prices reflect inter-dealer prices, without retail mark-up, markdown or commission.
58
On March 17, 2016, the last sales price per share of our Common Stock on the OTCQB was $0.03.
Penny Stock
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a market price of less than $5.00, other than securities registered on certain national securities exchanges or quoted on the NASDAQ system, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock, to deliver a standardized risk disclosure document prepared by the SEC, that: (a) contains a description of the nature and level of risk in the market for penny stocks in both public offerings and secondary trading; (b) contains a description of the broker’s or dealer’s duties to the customer and of the rights and remedies available to the customer with respect to a violation of such duties or other requirements of the securities laws; (c) contains a brief, clear, narrative description of a dealer market, including bid and ask prices for penny stocks and the significance of the spread between the bid and ask price; (d) contains a toll-free telephone number for inquiries on disciplinary actions; (e) defines significant terms in the disclosure document or in the conduct of trading in penny stocks; and (f) contains such other information and is in such form, including language, type size and format, as the SEC shall require by rule or regulation.
The broker-dealer also must provide, prior to effecting any transaction in a penny stock, the customer with (a) bid and offer quotations for the penny stock; (b) the compensation of the broker-dealer and its salesperson in the transaction; (c) the number of shares to which such bid and ask prices apply, or other comparable information relating to the depth and liquidity of the market for such stock; and (d) a monthly account statement showing the market value of each penny stock held in the customer’s account.
In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from those rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written acknowledgment of the receipt of a risk disclosure statement, a written agreement as to transactions involving penny stocks, and a signed and dated copy of a written suitability statement.
These disclosure requirements may have the effect of reducing the trading activity for our common stock. Therefore, stockholders may have difficulty selling our securities.
As of March 18, 2016, we had 81 record holders of our common stock holding 584,531,788 shares of common stock.
We have not paid any cash dividends to our shareholders. The declaration of any future cash dividends is at the discretion of our Board and depends upon our earnings, if any, our capital requirements and financial position, and general economic conditions. It is our present intention not to pay any cash dividends in the foreseeable future, but rather to reinvest earnings, if any, in our business operations.
Securities authorized for issuance under equity compensation plans
Not applicable.
59
The transfer agent for our common stock is Corporate Stock Transfer, Inc. located at 3200 Cherry Creek South Drive, Suite 430, Denver CO. 80209 and its telephone number is (303) 282-4800.
FINANCIAL STATEMENTS
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of the results of operations and financial condition for the three and six months ended December 31, 2015 and 2014 (unaudited) and the fiscal years ended June 30, 2015 and 2014 should be read in conjunction with our consolidated financial statements and the notes to those consolidated financial statements that are included elsewhere in this Registration Statement. Our discussion includes forward-looking statements based upon current expectations that involve risks and uncertainties, such as our plans, objectives, expectations and intentions. Actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a result of a number of factors. See “Forward-Looking Statements.”
Overview
We are a development stage healthcare company that is currently focused on developing new cancer treatments for patients, suffering from pancreatic, ovarian and colorectal cancers. Together with our scientific and oncology consultants, we have developed a rational, composite formulation of anti-cancer compounds, which together exert a number of effects designed to control or prevent tumors from recurring and spreading through the body. Our leading products are variations upon our novel formulation and involve or employ pro-enzymes, which are inactive precursors of enzymes. As a result of positive early indications of the anti-cancer effects of our technology, we intend to submit our pro-enzyme treatment to the rigorous, formal non-clinical and clinical development and trial processes required to obtain the regulatory approval necessary to commercialize it and any product(s) derived and/or to be derived therefrom.
In the near term, we intend to target patients with limited remaining therapeutic options for the treatment of solid tumors such as pancreatic, ovarian and colorectal tumors. In the future, we intend to development our lead product to treat (i) early stage cancer and (ii) pre-cancerous diseases and (iii) as a preventative measure for patients at risk of developing cancer based on genetic screening.
Critical Accounting Estimates
Below the Company will provide a discussion of its more subjective accounting estimation processes for purposes of (i) explaining the methodology used in calculating the estimates, (ii) the inherent uncertainties pertaining to such estimates, and (iii) the possible effects of a significant variance in actual experience, from that of the estimate, on the Company’s financial condition. Estimates involve the employ of numerous assumptions that, if incorrect, could create a material adverse impact on the Company’s results of operations and financial condition.
Foreign Currency Translation and Comprehensive Income (Loss): The Company’s functional currency is the Australian dollar (AUD). For financial reporting purposes, the Australian dollar has been translated into United States dollars ($) and/or USD as the reporting currency. Assets and liabilities are translated at the exchange rate in effect at the balance sheet date. Revenues and expenses are translated at the average rate of exchange prevailing during the reporting period. Equity transactions are translated at each historical transaction date spot rate. Translation adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’ equity (deficit) as “accumulated other comprehensive income (loss).” Gains and losses resulting from foreign currency transactions are included in the statement of operations and comprehensive loss as other income (expense).
60
Accounting for Income Taxes: The Company is governed by Australia and United States income tax laws, which are administered by the Australian Taxation Office and the United States Internal Revenue Service, respectively. The Company follows FASB ASC 740 when accounting for income taxes, which requires an asset and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed annually for temporary differences between the financial statements and tax bases of assets and liabilities that will result in taxable or deductible amounts in the future based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Income tax expense is the tax payable or refundable for the period plus or minus the change during the period in deferred tax assets and liabilities.
The Company adopted provisions of ASC 740, Sections 25 through 60, “Accounting for Uncertainty in Income Taxes." These sections provide detailed guidance for the financial statement recognition, measurement and disclosure of uncertain tax positions recognized in the financial statements. Tax positions must meet a “more-likely-than-not” recognition threshold at the effective date to be recognized upon the adoption of ASC 740 and in subsequent periods.
Accounting for Stock Based Compensation: The Company records stock based compensation in accordance with ASC section 718, “Stock Compensation” and Staff Accounting Bulletin (SAB) No. 107 (SAB 107) issued by the Securities and Exchange Commission (SEC) in March 2005 regarding its interpretation of ASC 718. ASC 718 requires the fair value of all stock-based employee compensation awarded to employees to be recorded as an expense over the related requisite service period. The statement also requires the recognition of compensation expense for the fair value of any unvested stock option awards outstanding at the date of adoption. The Company values any employee or non-employee stock based compensation at fair value using the Black-Scholes Option Pricing Model.
The Company accounts for non-employee share-based awards in accordance with the measurement and recognition criteria of ASC 505-50 "Equity-Based Payments to Non-Employees.
Derivative Instruments: ASC Topic 815, Derivatives and Hedging (“ASC Topic 815”), establishes accounting and reporting standards for derivative instruments and for hedging activities by requiring that all derivatives be recognized in the balance sheet and measured at fair value. Gains or losses resulting from changes in the fair value of derivatives are recognized in earnings or recorded in other comprehensive income (loss) depending on the purpose of the derivatives and whether they qualify and have been designated for hedge accounting treatment. The Company does not have any derivative instruments for which it has applied hedge accounting treatment.
Convertible Notes With Variable Conversion Options: The Company has entered into convertible notes, some of which contain variable conversion options, whereby the outstanding principal and accrued interest may be converted, by the holder, into common shares at a fixed discount to the price of the common stock at the time of conversion. The Company treats these convertible notes as stock settled debt under ASC 480 and measures the fair value of the notes at the time of issuance, which is the result of the share price discount at the time of conversion, and records the put premium as accretion to interest expense to the date of first conversion.
Research and Development Tax Credits: The Company may apply for Research and Development tax concessions with the Australian Taxation Office on an annual basis. Although the amount is possible to estimate at year end, the Australian Taxation Office may reject or materially alter the claim amount. Accordingly, the Company does not recognize the benefit of the claim amount until cash receipt since collectability is not certain until such time. The tax concession is a refundable credit. If the Company has net income then the Company can receive the credit which reduces its income tax liability. If the Company has net losses then the Company may still receive a cash payment for the credit, however, the Company's net operating loss carry forwards are reduced by the gross equivalent loss that would produce the credit amount when the income tax rate is applied to that gross amount. The concession is recognized as an income tax benefit, in operations, upon receipt.
Recent Accounting Pronouncements
61
In August 2014, the FASB issued ASU 2014-15, “Presentation of Financial Statements – Going Concern (Topic 205-40)”, which requires management to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern for each annual and interim reporting period. If substantial doubt exists, additional disclosure is required. This new standard will be effective for the Company for annual and interim periods beginning after December 15, 2016. Early adoption is permitted. The Company expects to adopt this new standard as of December 31, 2016 and the Company will continue to assess the impact on its consolidated financial statements.
On May 8, 2015, the FASB issued ASU 2015-08, “Business Combinations (Topic 805) Pushdown Accounting ” which conforms the FASB’s guidance on pushdown accounting with the SEC’s guidance. ASU 2015-08 is effective for annual periods beginning after December 15, 2015. As of December 31, 2015, this ASU has not had a material impact on the unaudited consolidated financial statements.
In April 2015, the Financial Accounting Standards Board issued Accounting Standards Update No. 2015-03, "Simplifying the Presentation of Debt Issuance Costs," which changes the presentation of debt issuance costs in financial statements. Under this guidance such costs would be presented as a direct deduction from the related debt liability rather than as an asset. This guidance is effective for interim and annual reporting periods beginning after December 15, 2015. As of December 31, 2015, this ASU has not had a material impact on the unaudited consolidated balances current presentation.
Financial Accounting Standards Board, Accounting Standard Updates which are not effective until after December 31, 2015 are not expected to have a significant effect on the Company’s consolidated financial position or results of operations.
Results of Operations
The following discussion should be read in conjunction with the consolidated financial statements and notes thereto included elsewhere in this form S-1. The results discussed below are of the Company and its wholly-owned Australian subsidiary, Propanc PTY Ltd.
For the Three Months Ended December 31, 2015 compared to the Three Months Ended December 31, 2014
Revenue
For the three months ended December 31, 2015 and 2014, we generated no revenue because we are currently undertaking research and development activities for market approval and there were no sales generated in this period.
Administration Expense
Administration expenses increased to $996,129 for the three months ended December 31, 2015 as compared with $244,431 for the three months ended December 31, 2014. This increase is primarily attributable to an increase in stock based expenses of approximately $340,000, an increase in wages and benefits of approximately $70,000 and an increase in professional fees of approximately $300,000 during the three months ended December 31, 2015 as compared to the three months ended December 31, 2014.
Occupancy Expense
Occupancy expense increased by approximately $2,300 to $4,889 for the three months ended December 31, 2015. On May 1, 2015, we moved to new premises. On May 1, 2015, we entered into a month to month lease agreement with new landlord with a monthly rental fee of approximately $2,200 AUD. The increase is primarily attributable to an increase in monthly rent expense as compared to prior year period.
62
Research and Development Expenses
Research and development was $142,803 for the three months ended December 31, 2015 as compared with $2,443 for the three months ended December 31, 2014. Research and development expenditures are primarily attributable to completing animal efficacy models on PRP and to completing the manufacturing, production of drug substance and product for preclinical and clinical trials, as well as undertaking formal toxicology studies and non clinical development. We are looking to raise sufficient capital to undertake the next stage of development for our current programs. We continue to expend our efforts to continue creating value by completing our patent filings and publishing our scientific discoveries, and we are negotiating with third parties to assist with raising the capital needed to complete our planned research and development activities.
Interest Expense/Income
Interest expense increased to $1,155,645 for the the three months ended December 31, 2015 as compared with $100,176 for the three months ended December 31, 2014. Interest expense is primarily comprised of $775,000 debt discount amortization, and $325,000 accretion of debt premium. This increase is primarily attributable to an increased debt discounts of convertible notes issued by the Company during the three months ended December 31, 2015.
Change in Fair Value of Derivative Liabilities
Change in fair value of derivative liabilities decreased to a loss of $(1,347,743) for the three months ended December 31, 2015 as compared with a gain of $8,206 for the three months ended December 31, 2014. This decrease is primarily attributable to an increase in the issuance of convertible notes with repricing options and variable conversion pricing and a decrease in our stock price during the three months ended December 31, 2015.
Foreign Currency Transaction Gain (Loss)
Foreign currency transaction gain (loss) increased to a gain of $72,035 for the three months ended December 31, 2015 as compared with a loss of $157,655 for the three months ended December 31, 2014. The increase in foreign currency transaction gain (loss) is primarily attributable to a stronger US Dollar versus the Australian Dollar and increased operating activities in Australia during the three months ended December 31, 2015 as compared to the three months ended December 31, 2014.
Net loss
Net loss increased to $3,562,067 for the three months ended December 31, 2015 as compared with $345,479 for the three months ended December 31, 2014. The increase is primarily attributable to an increase in operating expenses of approximately $894,000, an increase in interest expense of approximately $1,100,000, and an increase in the loss related to a change in fair value of derivative liability of approximately $1,350,000 offset by an increase in foreign currency gain of approximately $230,000.
For the Six Months Ended December 31, 2015 compared to the Six Months Ended December 31, 2014
Revenue
For the six months ended December 31, 2015 and 2014, we generated no revenue because we are currently undertaking research and development activities for market approval and there were no sales generated in this period.
Administration Expense
Administration expenses increased to $1,845,108 for the six months ended December 31, 2015 as compared with $475,913 for the six months ended December 31, 2014. This increase is primarily attributable to an increase in stock based expenses of approximately $750,000, an increase in wages and benefits of $112,000 and an increase in professional fees of approximately $570,000 during the six months ended December 31, 2015 as compared to the six months ended December 31, 2014.
63
Occupancy Expense
Occupancy expense increased by approximately $4,500 to $9,827 for the six months ended December 31, 2015. On May 1, 2015, we moved to new premises. On May 1, 2015, we entered into a month to month lease agreement with new landlord with a monthly rental fee of approximately $2,200 AUD. The increase is primarily attributable to an increase in monthly rent expense as compared to prior year period.
Research and Development Expenses
Research and development was $296,277 for the six months ended December 31, 2015 as compared with $6,322 for the six months ended December 31, 2014. Research and development expenditures are primarily attributable to completing animal efficacy models on PRP and to completing the manufacturing, production of drug substance and product for preclinical and clinical trials, as well as undertaking formal toxicology studies and non clinical development. We are looking to raise sufficient capital to undertake the next stage of development for our current programs. We continue to expend our efforts to continue creating value by completing our patent filings and publishing our scientific discoveries, and we are negotiating with third parties to assist with raising the capital needed to complete our planned research and development activities.
Interest Expense/Income
Interest expense increased to $1,574,289 for the the six months ended December 31, 2015 as compared with $648,655 for the six months ended December 31, 2014. Interest expense is primarily comprised of $961,000 debt discount amortization, and $562,000 accretion of debt premium. This increase is primarily attributable to an increased debt discounts of convertible notes issued by the Company during the six months ended December 31, 2015.
Change in Fair Value of Derivative Liabilities
Change in fair value of derivative liabilities decreased to a loss of $(551,890) for the six months ended December 31, 2015 as compared with a gain of $122,742 for the six months ended December 31, 2014. This decrease is primarily attributable to an increase in the issuance of convertible notes with repricing options and variable conversion pricing and a decrease in our stock price during the six months ended December 31, 2015.
Foreign Currency Transaction Loss
Foreign currency transaction loss decreased to $138,704 for the six months ended December 31, 2015 as compared with $182,612 for the six months ended December 31, 2014. The decrease in foreign currency transaction loss is primarily attributable to a stronger US Dollar versus the Australian Dollar and increased operating activities in Australia during the six months ended December 31, 2015 as compared to the six months ended December 31, 2014.
Net loss
Net loss increased to $4,400,961 for the six months ended December 31, 2015 as compared with $1,128,779 for the six months ended December 31, 2014. The increase is primarily attributable to an increase in operating expenses of approximately $1,660,000, an increase in interest expense of approximately $984,000, and an increase in the loss related to a change in fair value of derivative liability of approximately $675,000.
For the Fiscal Year Ended June 30, 2015 compared to the Fiscal Year Ended June 30, 2014
Revenue
For the fiscal years 2015 and 2014 we generated no revenue because we are currently undertaking research and development activities for market approval and there were no sales generated in this period.
Administration Expense
Administration expense increased to $1,567,549 for the year ended June 30, 2015 as compared with $742,037 for the year ended June 30, 2014. This increase is primarily attributable to an increase in stock based expenses of approximately $160,000 and an increase in capital raising expenses of $130,000 and an increase in professional fees of approximately $450,000 during the year ended June 30, 2015 as compared to the year ended June 30, 2014.
64
Occupancy Expense
Occupancy expense decreased by approximately $7,300 to $3,719 for the year ended June 30, 2015. From July 2013 through April 30, 2015, we utilized office space at a certain location. There was no formal lease agreement and no amounts were paid, but we had accrued a liability as of April 30, 2015 of approximately $21,000 in anticipation of a month to month agreement retroactive to July 1, 2013 at approximately $1,000 per month. On May 1, 2015, we moved to new premises. The prior landlord verbally agreed that he would not be pursuing payment of any outstanding rent due, therefore we reversed the accrued rent liability and recorded a gain. On May 1, 2015, we entered into a month to month lease agreement with new landlord with a monthly rental fee of approximately $2,200 AUD. The decrease is primarily attributable to only recording two months of rent expense.
Research and Development Expenses
Research and development was $134,319 for the year ended June 30, 2015 as compared with $8,168 for the year ended June 30, 2014. Research and development expenditures are primarily attributable to completing animal efficacy models on PRP and to completing the manufacturing, production of drug substance and product for preclinical and clinical trials, as well as undertaking formal toxicology studies and non clinical development. We are looking to raise sufficient capital to undertake the next stage of development for our current programs. We continue to expend our efforts to continue creating value by completing our patent filings and publishing our scientific discoveries, and we are negotiating with third parties to assist with raising the capital needed to complete our planned research and development activities.
Interest Expense/Income
Interest expense increased to $1,323,902 for the year ended June 30, 2015 as compared with $93,147 for the year ended June 30, 2014. Interest expense is primarily comprised of $355,000 premium of the liability agreement, $25,000 premium of a convertible note, $102,000 face interest, $83,000 debt discount amortization, and $600,000 accretion of debt premium. This increase is primarily attributable to the increased number of interest bearing loans made to the Company during the fiscal year.
Change in Fair Value of Derivative Liabilities
Change in fair value of derivative liabilities increased to $541,981 for the year ended June 30, 2015 as compared with $16,522 for the year ended June 30, 2014. This increase is primarily attributable to an increase in the issuance of convertible notes with repricing options and variable conversion pricing and an increase in our stock price during the fiscal year.
Gain on Debt Settlements, Net
Gain on debt settlements increased to $375,547 for the year ended June 30, 2015 as compared with $0 for the year ended June 30, 2014. The increase in gain on debt settlements is primarily attributable to a gain of approximately $402,000 for write down of premiums and fees upon termination of the liability agreement, a gain of approximately $51,500 in connection with the Settlement and Stipulation Agreement and a gain of approximately $9,000 in connection with the reversal of the rent liability, offset by a loss of approximately $86,500 in connection with the Debt Settlement Agreement with some of our directors.
Foreign Currency Transaction Loss
Foreign currency transaction loss increased to $244,332 for the year ended June 30, 2015 as compared with $6,959 for the year ended June 30, 2014. The increase in foreign currency transaction loss is primarily attributable to a stronger US Dollar versus the Australian Dollar and increased operating activities in Australia during year ended June 30, 2015 as compared to year ended June 30, 2014.
65
Income Tax Benefit
During the years ended June 30, 2015 and 2014, the Company applied for and received from the Australian Taxation Office a research and development tax credit in the amount of $77,470 and $48,267 respectively.
Net loss
Net loss increased to $3,412,754 for the year ended June 30, 2015 as compared with $829,564 for the year ended June 30, 2014. The increase is primarily attributable to an increase in operating expenses of approximately $950,000, an increase in interest expense of approximately $1,200,000, an increase in foreign currency loss of approximately $230,000, an increase in the loss related to a change in fair value of derivative liability of approximately $520,000 and an increase in other expenses of approximately $50,000, offset by an increase in gain on debt settlements of approximately $375,000, and an increase in income tax benefit of approximately $30,000.
Liquidity and Capital Resources
| For the Six Months Ended December 31, |
||||||||
| 2015 | 2014 | |||||||
| Net cash used in operating activities | $ | (1,488,222 | ) | $ | (271,996 | ) | ||
| Net cash used in investing activities | $ | (676 | ) | $ | - | |||
| Net cash provided by financing activities | $ | 2,468,172 | $ | 99,000 | ||||
| For the Fiscal Year Ended June 30, |
||||||||
| 2015 | 2014 | |||||||
| Net cash used in operating activities | $ | (1,426,479 | ) | $ | (226,442 | ) | ||
| Net cash used in investing activities | $ | (5,585 | ) | $ | - | |||
| Net cash provided by financing activities | $ | 1,282,045 | $ | 311,141 | ||||
Net Cash Used in Operations
Net cash used in operations was $1,488,222 for the six months ended December 31, 2015 compared to $271,996 for the six months ended December 31, 2014. This increase was primarily attributable to an increase in the net loss of approximately $3,300,000 offset by an increase in common stock issued for services of approximately $1,000,000, increase in loss on change in derivative liability of approximately $675,000 offset by an increase in accretion of put premiums and amortization of debt discount of approximately $1,100,000.
Net cash used in operations was $1,426,479 for the fiscal year ended June 30, 2015 compared to $226,442 for the fiscal year ended June 30, 2014. This increase was primarily attributable to an increase in net loss of approximately $2,600,000 and an increase in gain on settlement of approximately $375,000 offset by an increase in accretion of put premiums and amortization of debt discount of approximately $1,260,000 and an increase in change in derivative liability of approximately $520,000.
Net Cash Used in Investing Activities
Net cash used in investing activities was $676 for the six months ended December 31, 2015 compared to $0 for the six months ended December 31, 2014. This increase was primarily attributable to the purchase of equipment during the six months ended December 31, 2015.
66
Net cash used in investing activities was $5,585 for the fiscal year ended June 30, 2015 compared to $0 for the fiscal year ended June 30, 2014. This increase was primarily attributable to the purchase of equipment and payment of security deposit during the fiscal year ended June 30, 2015.
Cash Flows Provided by Financing Activities
Cash flows provided by financing activities for the six months ended December 31, 2015 were $2,468,172 compared to $99,000 for the six months ended December 31, 2014. During the six months ended December 31, 2015, we had proceeds from convertible promissory notes of approximately $3,000,000 offset by repayments of convertible promissory notes of approximately $464,000 and loan repayments to principal stockholder and others of approximately $45,000.
Cash flows provided by financing activities for the fiscal year ended June 30, 2015 were $1,282,045 compared to $311,141 for the fiscal year ended June 30, 2014. During the year ended June 30, 2015, we had proceeds from convertible promissory notes of $1,438,500 and proceeds from issuance of common stock for cash of $29,000 offset by repayments of convertible promissory notes of $157,000 and loan repayments to principal stockholder of approximately $28,500.
We have substantial capital resource requirements and have incurred significant losses since inception. As of December 31, 2015, we had $1,168,118 in cash. Based upon our current business plans, we will need considerable cash investments to be successful. Although such capital requirements are in excess of what we have in available cash, we recently raised a significant debt financing of approximately $4,000,000 which should give us enough available cash to meet our obligations over the next 12 months.
Related Party Transactions
Since inception, we have conducted transactions with directors and director related entities. These transactions included the following:
As of December 31, 2015 and June 30, 2015, the Company owed certain directors a total of $54,005 and $79,416 respectively, for money loaned to the Company throughout the years.
As of December 31, 2015 and June 30 2015, the Company owed two directors a total of $33,471 and $35,108, respectively, related to expenses paid on behalf of the Company related to corporate startup costs and intellectual property.
Going Concern Qualification
The Company has incurred significant losses and cash used in operations, and such losses and use of cash are expected to continue. The Company’s Independent Registered Public Accounting Firm has included a "Going Concern Qualification" in their report for the fiscal years ended June 30, 2015 and 2014. In addition, the Company has negative working capital and no revenues. The foregoing raises substantial doubt about the Company's ability to continue as a going concern. Management's plans include seeking additional capital or debt financing. There is no guarantee that additional capital or debt financing will be available when and to the extent required, or that if available, it will be on terms acceptable to the Company. The unaudited consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. The "Going Concern Qualification" might make it substantially more difficult to raise capital.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources.
67
Changes In and Disagreements with Accountants on Accounting and Financial Disclosure
There have been no changes in or disagreements with accountants on accounting or financial disclosure matters.
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
Our directors, executive officers and key employees are listed below. The number of directors is determined by our board of directors. All directors hold office until the next annual meeting of the board or until their successors have been duly elected and qualified. Officers are elected by the board of directors and their terms of office are, except to the extent governed by employment contract, at the discretion of the board of directors.
| Name | Age | Principal Positions With Us | ||
| James Nathanielsz | 41 | Chief Executive Officer, Secretary, Treasurer and Director | ||
| Dr. Julian Kenyon | 68 | Director |
Set forth below is a brief description of the background and business experience of our director and executive officer for the past five years.
James Nathanielsz has served as a director since inception. Mr. Nathanielsz has served as a director and Chief Executive Officer of our Australian company since October 2007. From July 2006 until October 2007, Mr. Nathanielsz served as the New Products Manager of Biota Holdings Limited, an anti-infective drug development company in Australia. Mr. Nathanielsz was selected as a director because he is the Co-Founder of our Australian company and for his experience in R&D and manufacturing and distribution. Mr. Nathanielsz graduated with a Bachelor of Applied Science, majoring in Biochemistry/Applied Chemistry and subsequently with a Master of Entrepreneurship & Innovation from Swinburne University of Technology in Melbourne, Australia.
Dr. Julian Kenyon has served as a director since inception. Dr. Kenyon founded our Australian company and was appointed as a director of our Australian company on February 12, 2008. Since 2000, Dr. Kenyon has served as an integrated medical physician and Medical Director of the Dove Clinic for Integrated Medicine in Winchester and London. Dr. Kenyon is the Founder-Chairman of the British Medical Acupuncture Society in 1980 and Co-Founder of the Centre for the Study of Complementary Medicine in Southampton and London. Dr. Kenyon was selected as a director because he is the Co-Founder of the Australian subsidiary and the business is based on his initial work at the Dove Clinic. Dr. Kenyon graduated from the University of Liverpool with a Bachelor of Medicine and Surgery and subsequently with a research degree, Doctor of Medicine. Since 1972, he was appointed a Primary Fellow of the Royal College of Surgeons, Edinburgh.
Family Relationships
There are no family relationships between Mr. Nathanielsz and Dr. Kenyon.
Involvement in Certain Legal Proceedings
To the best of our knowledge, none of directors or officers, during the past ten years:
| ● | been convicted in a criminal proceeding or been subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| ● | had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation or business association of which he was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time; |
| ● | been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity; |
| ● | been found by a court of competent jurisdiction in a civil action or by the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
68
| ● | been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| ● | been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Except as set forth in our discussion below in “Certain Relationships and Related Transactions,” none of our directors or executive officers has been involved in any transactions with us or any of our directors, executive officers, affiliates or associates which are required to be disclosed pursuant to the rules and regulations of the SEC.
Term of Office
Our directors hold office until a successor is elected and qualified or until earlier of resignation, removal from office or death.
Board Committees
Our Board of Directors has no separate committees and our Board of Directors acts as the audit committee and the compensation committee. We do not have an audit committee financial expert serving on our Board of Directors.
Scientific Advisory Board
We have a Scientific Advisory Board that provides advice relating to the following:
| · | The identification, assessment, evaluation, selection, conduct and management of research projects, both those which are under review and are in progress; | |
| · | Intellectual property; and | |
| · | Commercialization. |
The Scientific Advisory Board may also address issues related to improving project selection, formal review processes and management procedures within Propanc Health Group. The Scientific Advisory Board will generally be composed of an advisory panel of clinicians with expertise in translational research.
As of March 22, 2016, the members of the Scientific Advisory Board were:
| · | Professor John Smyth | |
| · | Professor Klaus Kutz (Also serving as Chief Medical Officer of the Company) | |
| · | Dr. Ralf Brandt | |
| · | Dr. Joseph Chalil |
Each of the members of our Scientific Advisory Board acts as an independent consultant and is compensated on an hourly basis for his services. There is presently no stock based compensation for their services.
Professor Kutz is also acting as Chief Medical Officer for Propanc, His compensation continues to be based on an hourly rate as per his Advisory Board Agreement. Propanc intends to appoint Professor Kutz as Chief Medical Officer for Propanc in a full time capacity at a time which is mutually agreed upon between both parties.
69
Professor John Smyth
John Smyth has for the past 25 years served as Chair of Medical Oncology in the University of Edinburgh Medical School, where his major research interest is the development and evaluation of new anti-cancer drugs. He has published over 300 papers and is Editor-in-Chief of the European Journal of Cancer. He served for several years on the UK Committee on Safety of Medicines; currently Chair's the Expert Advisory Group for Oncology & Haematology for the Commission on Human Medicines and serves on the Expert Oncology Advisory Group to the European Drug Licensing Board. He is a fellow of the Royal College of Physicians of Edinburgh and London, and fellow of the Royal Society of Edinburgh. He is a past-president of the European Society of Medical Oncology and was from 2005 - 2007 President of the Federation of European Cancer Societies.
Professor Klaus Kutz
Professor Kutz has fifteen years of experience as independent consultant in Clinical Pharmacology and Safety for pharmaceutical companies and clinical research organizations. His specialty over the last six years is Oncology, including preparation of multiple NDAs and INDs for small and medium sized pharmaceutical companies. He has prepared, organized and reported clinical Phase I studies in oncology and Phase II studies in different cancer indications (prostate, gastric, ovarian, small cell lung cancer) and Non-Hodgkin Lymphomas. Professor Kutz has more than 12 years of experience as Head of Clinical Pharmacology with world-wide responsibilities for Phase I and Clinical Pharmacokinetics in two internationally operating pharmaceutical companies, setting up and restructuring international Clinical Pharmacology departments. His achievements include the successful world-wide registration of multiple important Sandoz’ compounds by preparation of multiple NDAs (New Drug Applications) and Expert reports (including Written Summary), as well as the preparation of multiple INDs (Investigational New Drug Applications) for Sandoz Pharma Ltd and Sanofi Research. A specialist for Internal Medicine, Gastroenterology, and Clinical Pharmacology, he is also Professor of Medicine at the University of Bonn, Germany.
Dr. Ralf Brandt
Dr. Brandt is the co-founder of vivoPharm. He is a biochemist and cell biologist with over 15 years of experience in research programs of experimental oncology. Furthermore, he has immense experience in in vivo pharmacology and anti-cancer drug profiling. He received his License (BSc in Biochemistry and Animal Physiology) in 1986, and his PhD (in Biochemistry) in 1991 from the Martin-Luther University of Halle-Wittenberg, Germany. Dr. Brandt was employed at research positions at the National Cancer Institute in Bethesda, MD, USA and at Schering AG, Germany. Since 1990, Dr. Brandt has been active in the field of preclinical oncology. He led the Tumour Biology program at Novartis Pharma AG, Switzerland and established several transgenic mouse lines developing tumors under the control of oncogenes. During Dr. Brandt's long career in the pharmaceutical industry he has acquired significant knowledge and expertise in leading business units and representation of services to the pre-clinical research market. Dr. Brandt is a member of the Scientific Advisory Board at Receptor Inc. in Toronto Canada.
Dr. Joseph Chalil
Dr. Chalil is a Physician and Executive at Boehringer Ingelheim, the world's largest privately held pharmaceutical company. Headquartered in Ingelheim, Germany, Boehringer Ingelheim operates globally with 146 affiliates and a total of more than 47,700 employees. In 2014, Boehringer Ingelheim achieved net sales of about 13.3 billion euros. R&D expenditure corresponds to 19.9 per cent of its net sales.
In addition to his responsibilities at Boehringer Ingelheim, Dr. Chalil is the Chairman of Global Clinical Research and Trial Network of the American Association of Physicians of Indian Origin (AAPI) and has served as Scientific Advisor to AAPI for the past five years. AAPI is the second largest physician organization in the US second only to AMA, and the largest ethnic medical organization in the country.
A veteran of the United States Navy Medical Corps, Dr. Chalil is also board certified in healthcare management, and has been awarded Fellowship by the American College of Healthcare Executives, an international professional society of more than 40,000 healthcare executives who lead hospitals, healthcare systems and other healthcare organizations.
70
Dr. Chalil is an expert in U.S. Healthcare policy and a strong advocate for patient centered care, and has also served as an advisor to various national political campaigns on healthcare issues. Dr. Chalil completed his higher studies in University of Medicine and Dentistry of New Jersey, Davenport University, JJM Medical College and Baylor College of Medicine. He has been a Visiting Professor at various Universities and serves on various company Boards.
Code of Ethics
The Board is currently reviewing a Code of Conduct and Ethics (the “Code”) to apply to all of our directors, officers and employees. The Code is intended to promote ethical conduct and compliance with laws and regulations, to provide guidance with respect to the handling of ethical issues, to implement mechanisms to report unethical conduct, to foster a culture of honesty and accountability, to deter wrongdoing and to ensure fair and accurate financial reporting. Upon approval by the Board, a copy of the Code will be available at our website www.propanc.com.
Shareholder Communications
Although we do not have a formal policy regarding communications with the Board, shareholders may communicate with the Board by writing to us at Level 2, 555 Riversdale Road, Camberwell, VIC, Australia, Attention: Corporate Secretary, or by facsimile +61 (0)3 9882 9969. Shareholders who would like their submission directed to a member of the Board may so specify, and the communication will be forwarded, as appropriate.
Board Diversity
While we do not have a formal policy on diversity, our Board considers diversity to include the skill set, background, reputation, type and length of business experience of our Board members as well as a particular nominee’s contributions to that mix. Our Board believes that diversity brings a variety of ideas, judgments and considerations that benefit Propanc and our shareholders. Although there are many other factors, the Board seeks individuals with experience in business, financial and scientific research and development.
Board Assessment of Risk
Our risk management function is overseen by our Board. Our management keeps our Board apprised of material risks and provides our directors access to all information necessary for them to understand and evaluate how these risks interrelate, how they affect Propanc, and how management addresses those risks. Mr. Nathanielsz, as our Chief Executive Officer works closely together with the Board once material risks are identified on how to best address such risk. If the identified risk poses an actual or potential conflict with management, our independent directors may conduct the assessment. Presently, the primary risks affecting Propanc is the lack of working capital, the inability to generate sufficient revenues so that we have positive cash flow from operations and success of future clinical trials. The Board focuses on these key risks at each meeting and actively interfaces with management on seeking solutions.
Summary Compensation Table
The following table sets forth the compensation paid or accrued by us to our Chief Executive Officer, Chief Financial Officer and each of our other officers for the years ended June 30, 2015 and 2014.
The compensation reported in the summary compensation table below is not necessarily indicative of how we will compensate our officers in the future. We expect that we will continue to review, evaluate and modify our compensation framework and the compensation of our officers could as the business develops.
71
| Name and Principal Position | Year | Salary ($) |
All Other Compensation ($) |
Total ($) |
||||||||||
| James Nathanielsz (1) | 2015 | 200,000 | 18,500 | (2) | 218,500 | |||||||||
| Chief Executive Officer, Chief Financial Officer, Chief Operating Officer | 2014 | 137,685 | 12,736 | (2) | 150,421 | |||||||||
| Dr. Klaus Kutz (3) | 2015 | 18,229 | - | 18,229 | ||||||||||
| Chief Medical Officer | 2014 | 7,936 | - | 7,936 | ||||||||||
(1) Under an employment agreement dated February 25, 2015, Mr. Nathanielsz receives a gross annual salary of $300,000 AUD per year. From August 15, 2010 through February 25, 2015, Mr. Nathanielsz received a gross annual salary of $150,000 AUD per year.
(2) Under the employment agreement with the Company, Mr. Nathanielsz receives a 9.25% contribution to a pension of which he is the beneficiary.
(3) Pursuant to that certain Scientific Advisory Board Member Agreement, dated May 24, 2008, Dr. Kutz is compensated on per diem basis for his services rendered until the Company secures sufficient funds to employ Dr. Kutz on full-time basis.
Narrative to Summary Compensation Table
Employment Agreement with James Nathanielsz
The Company and James Nathanielsz entered into an employment agreement as of February 25, 2015 (the “Nathanielsz Employment Agreement”) setting forth the terms and conditions of Mr. Nathanliesz employment as the Company’s President and Chief Executive Officer. The Nathanielsz Employment Agreement also contemplates that Mr. Nathanielsz serves as a member of the Board. The Nathanielsz Employment Agreement expires February 25, 2018; however, the term of the Nathanielsz Employment Agreement will automatically review for successive one-year periods unless either party provides 30 days’ prior written notice of its intent not to review.
The Nathanielsz Employment Agreement provides Mr. Nathanielsz with a base salary of $25,000 AUD per month ($300,000 AUD annually) and a monthly contribution to Mr. Nathanielsz’s pension equal to 9.25% of his monthly salary. Mr. Nathanielsz has the ability to convert any accrued but unpaid salary into Common Stock at the end of each fiscal year as a conversion price to be determined by Mr. Nathanielsz and the Company, which will in no event be lower than par value or higher than the closing bid price on the date of conversion. The Company has also agreed to pay Mr. Nathanielsz an annual discretionary bonus in an amount up to 200% of his annual base salary, which bonus shall be determined by the Board and based upon the performance of the Company.
Mr. Nathanielsz is entitled to 20 days of annual leave and 8 days of paid sick leave. Mr. Nathanielsz is also entitled to participate in employee benefits plans, fringe benefits and perquisites maintained by the Company for similarly situated executives of the Company.
In the event that the Company provides notice of non-renewal of the Nathanielsz Employment Agreement, the Company terminates Mr. Nathanielsz without cause (as defined in the Nathanielsz Employment Agreement) or Mr. Nathanielsz terminates his employment for good reason (as defined in the Nathanielsz Employment Agreement), the Company has agreed to pay Mr. Nathanielsz a severance payment in an amount equal to Mr. Nathanielsz’s base salary for the year of termination in addition to accrued but unpaid salary, reimbursement of expenses and certain other employee benefits as determined under the terms of the applicable plans (the “Accrued Amounts”). In the event that Mr. Nathanielsz provides notice of non-renewal of the Nathanielsz Employment Agreement, the Company terminates Mr. Nathanielsz for cause or Mr. Nathanielsz terminates his employment without good reason, Mr. Nathanielsz is only be entitled to the Accrued Amounts.
The Company has agreed to indemnify Mr. Nathanielsz for any liabilities, costs and expenses incurred in the event that he is made a party to a proceeding due to his roles with the Company, other than any proceeding initiated by Mr. Nathanielsz or the Company relating to any dispute with respect to the Nathanielsz Employment Agreement or Mr. Nathanielsz’s employment.
The foregoing description of the SAB Agreements is qualified in its entirety by reference to the provisions of the Nathanielsz Employment Agreement filed as Exhibit 10.10 to this Registration Statement, which is incorporated herein by reference.
72
Under the terms of the Nathanielsz Employment Agreement, Mr. Nathanielsz is also subject to certain restrictive covenants, including a 1 year non-compete.
Agreement with Dr. Klaus Kutz
The Company and Dr. Klaus Kutz entered into a Scientific Advisory Board Member Agreement, dated May 24, 2008 setting forth the terms under which Dr. Kutz serves as a member of the Company’s Scientific Advisory Board (the “Kutz Agreement”). The Kutz Agreement primarily contains terms relating to the protection of the Company’s intellectual property. However, the Kutz Agreement provides that Dr. Kutz is paid a rate of 300 EURO for each hour of service provided. Dr. Kutz is currently being compensated in accordance with the Kutz Agreement for services he provides to the Company as acting Chief Medical Officer.
The foregoing description of the Kutz Agreement is qualified in its entirety by reference to the provisions of the Form Scientific Advisory Board Member Agreement filed as Exhibit 10.12 to this Registration Statement, which is incorporated herein by reference.
Outstanding Equity Awards at Fiscal Year-End Table
There are no outstanding equity awards.
Compensation of Directors
Director Agreement with Julian Kenyon
The Company and Julian Kenyon entered into a Director Agreement as of February 25, 2015 setting forth the terms and conditions of Mr. Kenyon’s service as a director on the Board (the “Director Agreement”). Mr. Kenyon’s appointment term is three years ending on February 25, 2018; however, the term this term will automatically review for successive one-year periods unless either party provides 30 days’ prior written notice of its intent not to review.
Under the Director Agreement, Mr. Kenyon receives monthly consideration of $10,000 AUD ($120,000 AUD annualized). Mr. Kenyon has the ability to convert any accrued but unpaid compensation into Common Stock at the end of each fiscal year as a conversion price to be determined by Mr. Kenyon and the Company, which will in no event be lower than par value or higher than the closing bid price on the date of conversion.
In the event that the Company provides notice of non-renewal of the Director Agreement, the Company terminates Mr. Nathanielsz without cause (as defined in the Director Agreement) or Mr. Kenyon terminates his employment for good reason (as defined in the Director Agreement), the Company has agreed to pay Mr. Kenyon a severance payment in an amount equal to Mr. Kenyon’s base salary for the year of termination in addition to accrued but unpaid salary and reimbursement of expenses (the “Accrued Amounts”). In the event that Mr. Kenyon provides notice of non-renewal of the Director Agreement, the Company terminates Mr. Kenyon for cause or Mr. Kenyon terminates his employment without good reason, Mr. Kenyon is only be entitled to the Accrued Amounts.
The Company has agreed to indemnify Mr. Kenyon for any liabilities, costs and expenses incurred in the event that he is made a party to a proceeding due to his role with the Company, other than any proceeding initiated by Mr. Kenyon or the Company relating to any dispute with respect to the Director Agreement or Mr. Kenyon’s service as a director.
Under the terms of the Director Agreement, Mr. Kenyon is also subject to certain restrictive covenants, including a 1 year non-compete.
The foregoing description of the Director Agreement is qualified in its entirety by reference to the provisions of the Director Agreement filed as Exhibit 10.11 to this Registration Statement, which is incorporated herein by reference.
Other Director Compensation
Directors are reimbursed for reasonable expenses incurred in attending meetings and carrying out duties as board members.
Scientific Advisory Board Members Compensation
The Company has entered into Scientific Advisory Board Member Agreements with each of the members of its Scientific Advisory Board (the “SAB Agreements”). The SAB Agreements contain substantially similar terms and primarily relate to the protection of the Company’s intellectual property. The SAB Agreements also include provisions for the members’ compensation for the services performed as a member of the Scientific Advisory Board. Messrs. Kutz, Brandt and Smyth each are paid a monetary fee for each of service provided and Mr. Chalil received shares of Company common stock.
The foregoing description of the SAB Agreements is qualified in its entirety by reference to the provisions of the Form Scientific Advisory Board Member Agreement filed as Exhibit 10.12 to this Registration Statement, which is incorporated herein by reference.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth the number of shares of our voting stock beneficially owned, as of March 18, 2016 by (i) those persons known by Propanc to be owners of more than 5% of Propanc’s common stock, (ii) each director, (iii) our Named Executive Officer, and (iv) all executive officers and directors as a group:
73
| Title of Class | Name and Address of Beneficial Owner | Amount and Nature of Beneficial Owner(1) |
Percent of Class (1) |
|||||||
| Common Stock | James Nathanielsz 576 Swan Street Richmond, VIC, 3121, Australia (2) |
42,961,111 | 7.35 | % | ||||||
| Common Stock | Dr. Julian Kenyon Beechwood, Embley Lane East Wellow, Near Romsey, Hampshire, SO51 6DN, United Kingdom (3) |
28,466,534 | 4.87 | % | ||||||
| Common Stock | All directors and executive officers as a group | 71,427,645 | 12.22 | % | ||||||
| 5% Shareholders: | ||||||||||
| Common Stock | Putney Consultants Ltd. (4) | 30,089,614 | 5.15 | % | ||||||
| Common Stock | Delafield Investments Limited | 171,000,000 | 29.25 | % | ||||||
| (1) | Applicable percentages are based on 584,531,788 shares outstanding as of March 18, 2016, adjusted as required by rules of the SEC. Beneficial ownership is determined under the rules of the SEC and generally includes voting or investment power with respect to securities. Shares of common stock subject to options, warrants and convertible notes currently exercisable or convertible, or exercisable or convertible within 60 days are deemed outstanding for computing the percentage of the person holding such securities but are not deemed outstanding for computing the percentage of any other person. Unless otherwise indicated in the footnotes to this table, Propanc believes that each of the shareholders named in the table has sole voting and investment power with respect to the shares of common stock indicated as beneficially owned by them. |
| (2) | Represents shares of common stock held by North Horizon Investments Pty Ltd., a Nathanielsz Family Trust. Mr. Nathanielsz, a director and executive officer of the Company, has voting and investment power over these shares. |
| (3) | Represents shares of common stock held by Dr. Julian Kenyon, a director of the Company. |
| (4) | Dr. Douglas Mitchell, a former director and executive officer of the Company, has voting and investment power of Putney Consultants Ltd. |
TRANSACTIONS WITH RELATED PERSONS, PROMOTERS AND CERTAIN CONTROL PERSONS
Since inception, Propanc Health Group Corporation has conducted transactions with directors and director related entities. These transactions included the following:
As of December 31, 2015 and June 30, 2015, the Company owed certain directors a total of $54,005 and $79,416 respectively, for money loaned to the Company throughout the years.
As of December 31, 2015 and June 30 2015, the Company owed two directors a total of $33,471 and $35,108, respectively, related to expenses paid on behalf of the Company related to corporate startup costs and intellectual property.
74
The following table represents the transactions with related person for the years ending June 30, 2015 and 2014:
| Aggregate Amount of Principal Loaned |
Amount of Principal Paid |
Outstanding Balance as of June 30, |
||||||||||||||||||||||||
| Name of Related Party |
Affiliation | 2015 | 2014 | 2015 | 2014 | 2015 | 2014 | |||||||||||||||||||
| James Nathanielsz** |
Chief Executive Officer & Chairman of the Board |
$ | 0 | * | $ | 86,225 | * | $ | 34,000 | * | $ | 59,084 | * | $ | 14,278 | * | $ | 78,586 | * | |||||||
| Julian Kenyon** | Director | $ | - | $ | - | $ | - | $ | - | $ | 8,491 | * | $ | 13,686 | * | |||||||||||
| Douglas Mitchell |
Former Chief Executive Officer and Chairman of the Board | $ | - | $ | - | $ | - | $ | - | $ | 56,647 | * | $ | 69,703 | * | |||||||||||
* Loans were made in Australian Dollar and the difference of amount is due to the fluctuating currency exchange rate between U.S. Dollar and Australian Dollar.
** During 2015, the Company issued the directors an aggregate of 50,913,820 shares of common stock pursuant to debt settlement agreements where a combined principal amount of $40,828 was converted.
The loans of $79,416 and $161,975 as of June 30, 2015 and 2014, respectively, are reflected as loans from directors and officer – related parties in the consolidated balance sheets.
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION OF SECURITIES ACT LIABILITIES
Our directors and officers are indemnified as provided by the Delaware corporate law and our Bylaws. We have agreed to indemnify each of our directors and certain officers against certain liabilities, including liabilities under the Securities Act. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the provisions described above, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than our payment of expenses incurred or paid by our director, officer or controlling person in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
We have been advised that in the opinion of the SEC indemnification for liabilities arising under the Securities Act is against public policy as expressed in the Securities Act, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities is asserted by one of our directors, officers, or controlling persons in connection with the securities being registered, we will, unless in the opinion of our legal counsel the matter has been settled by controlling precedent, submit the question of whether such indemnification is against public policy to a court of appropriate jurisdiction. We will then be governed by the court’s decision.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of our common stock offered by this prospectus. For purposes of this section, the term registration statement means the original registration statement and any and all amendments including the schedules and exhibits to the original registration statement or any amendment. This prospectus, filed as part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules thereto as permitted by the rules and regulations of the SEC.
75
For further information about us and our common stock, you should refer to the registration statement, including the exhibits This prospectus summarizes provisions that we consider material of certain contracts and other documents to which we refer you. Because the summaries may not contain all of the information that you may find important, you should review the full text of those documents. The registration statement, including its exhibits and schedules, may be inspected and copied at the public reference facilities maintained by the SEC at 100 F. Street, N.E., Room 1580, Washington, D.C. 20549. You may obtain information on the operation of the public reference room by calling 1-202-551-8909. Copies of such materials are also available by mail from the Public Reference Branch of the SEC at 100 F Street, N.E., Room 1580, Washington, D.C. 20549 at prescribed rates. In addition, the SEC maintains a website at (http://www.sec.gov) from which interested persons can electronically access the registration statement, including the exhibits and schedules to the registration statement.
PROPANC HEALTH GROUP CORPORATION
171,000,000 SHARES OF COMMON STOCK
PROSPECTUS
YOU SHOULD RELY ONLY ON THE INFORMATION CONTAINED IN THIS DOCUMENT OR THAT WE HAVE REFERRED YOU TO. WE HAVE NOT AUTHORIZED ANYONE TO PROVIDE YOU WITH INFORMATION THAT IS DIFFERENT. THIS PROSPECTUS IS NOT AN OFFER TO SELL COMMON STOCK AND IS NOT SOLICITING AN OFFER TO BUY COMMON STOCK IN ANY STATE WHERE THE OFFER OR SALE IS NOT PERMITTED.
Until _____________, all dealers that effect transactions in these securities whether or not participating in this offering may be required to deliver a prospectus. This is in addition to the dealer’s obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
The Date of This Prospectus is March 24, 2016
PART II INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
| Securities and Exchange Commission Registration Fee | $ | 516.59 | ||
| Transfer Agent Fees* | $ | - | ||
| Accounting fees and expenses* | $ | 5,000.00 | ||
| Legal fees and expenses* | $ | 15,000.00 | ||
| Blue Sky fees and expenses* | $ | - | ||
| Total* | $ | 20,516.59 |
* Estimated
Item 14. Indemnification of Directors and Officers.
Our directors and officers are indemnified as provided by the Delaware corporate law and our Bylaws. We have agreed to indemnify each of our directors and certain officers against certain liabilities, including liabilities under the Securities Act. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the provisions described above, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than our payment of expenses incurred or paid by our director, officer or controlling person in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
76
We have been advised that in the opinion of the SEC indemnification for liabilities arising under the Securities Act is against public policy as expressed in the Securities Act, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities is asserted by one of our directors, officers, or controlling persons in connection with the securities being registered, we will, unless in the opinion of our legal counsel the matter has been settled by controlling precedent, submit the question of whether such indemnification is against public policy to a court of appropriate jurisdiction. We will then be governed by the court’s decision.
Item 15. Recent Sales of Unregistered Securities.
On September 30, 2013 the Company’s subsidiary issued a debenture for $139,683 (AUD$150,000) plus warrants for 3,000,000 common shares of the Company. The Company agreed to pay 12% interest on the principal amount and the maturity date is December 31, 2015. The debenture is convertible only at the Company’s option into common stock at $0.0698 per share and is convertible at that same rate by the lender only upon default by the Company, as defined in the debenture.
On May 29 and May 30, 2014 the Company issued six convertible promissory notes to certain investors for an aggregate amount of $400,000, $200,000 of which are paid for by an offsetting $200,000 promissory notes issued to the Company by the investors. The Company agreed to pay 8% interest on the principal amount and the maturity date is one year from the execution date of the notes. The notes are convertible into company’s common stock at any time after the requisite Rule 144 holding period, subject to certain terms and conditions, at a conversion price equal to 55% of the lowest trading bid price in the ten (10) trading days prior to the conversion.
On April 20, 2015, the Company issued a convertible note payable for $17,500. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is April 20, 2016. The note is convertible at the option of the holder at any time at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received.
On April 24, 2015, the Company received payment of the note receivable of $45,000, less the OID of $7,500 that offsets the back-end note that was issued on February 10, 2015. Proceeds from the note receivable of $2,250 were paid directly to legal fees resulting in net cash proceeds of $35,250 received by the Company. This back-end note is related to the initial convertible note that was issued on February 10, 2015 and has the same terms as previously discussed. As a result, the back-end note is now eligible for conversion at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received.
On April 24, 2015, the Company received payment of a second note receivable of $45,000, less the OID of $7,500 that offsets the back-end note that was issued on February 17, 2015. Proceeds from the note receivable of $2,250 were paid directly to legal fees resulting in net cash proceeds of $35,250 received by the Company. This back-end note is related to the initial convertible note that was issued on February 17, 2015 and has the same terms as previously discussed. As a result, the back-end note is now eligible for conversion at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received.
On April 27, 2015, the Company received payment of a third note receivable of $170,500, less the OID of $13,000 that offsets the back-end note that was issued on March 12, 2015. Proceeds from the note receivable of $7,500 were paid directly to legal fees resulting in net cash proceeds of $150,000 received by the Company. This back-end note is related to the initial convertible note that was issued on March 12, 2015 and has the same terms as previously discussed. As a result, the back-end note is now eligible for conversion at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received.
77
On May 19, 2015, the Company entered into a Securities Purchase Agreement (“SPA”), to issue a series of nine back end convertible notes in the principal sum of $782,500, pursuant to the SPA, the Company issued to the lender nine convertible promissory notes termed "Back-End Notes", in the amounts of $37,500 ("Back-End Note 1"), $37,500 ("Back-End Note 2"), $157,500 ("Back-End Note 3"), $150,000 ("Back-End Note 4"), $17,500 ("Back-End Note 5"), $37,500 ("Back-End Note 6"), $37,500 ("Back-End Note 7"), $157,500 ("Back-End Note 8") and $150,000 ("Back-End Note 9"). These notes have the same terms as the initial convertible notes. Each Back-End Note shall initially be paid for by an offsetting promissory note issued to the Company by the lender ("Note Receivable") provided that prior to the conversion of the Back-End Notes, the holders must have paid off the Notes Receivable in cash. Each Note Receivable is due on May 19, 2016, unless the Company does not meet the “current public information” requirement pursuant to Rule 144, in which case both the Back-End Notes and the Notes Receivable may both be cancelled. Each Note Receivable is initially secured by the pledge of the Back-End Notes, but may be exchanged for other collateral with an appraised value of at least the principal amount of the note less the OID, upon Company’s approval following a three (3) day written notice to the Company. The term of the Notes Receivable and the Back-End Notes are one year, upon which the outstanding principal and interest is payable. The amounts funded plus accrued interest under Back-End Notes are convertible into common stock at any time after the requisite Rule 144 holding period (subject to the condition above for the Back-End Notes), at a conversion price equal to 55% of the lowest trading bid price in the ten (10) trading days prior to the conversion.
On June 2, 2015, the Company received payment of a fourth Note Receivable of $150,000 that offsets the back-end note that was issued on March 20, 2015. Proceeds from the note receivable of $7,500 were paid directly to legal fees resulting in net cash proceeds of $142,500 received by the Company. This back-end note is related to the initial convertible note that was issued on March 20, 2015 and has the same terms as previously discussed. As a result, the back-end note is now eligible for conversion at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received.
On June 4, 2015, the Company executed a convertible promissory note in the principal sum of $1,215,000, with an OID of $110,000. The consideration to be paid to the lender shall be equal to the consideration actually paid by the lender plus prorated interest and any other fees that the Company shall be required to pay. The original issue discount shall also be prorated based on the actual consideration received to equal approximately 10% of the consideration received. The Company agreed to pay 10% interest per annum on the principal amount and the maturity date is ten months from the date of each payment to the Company, and is the date upon which the principal sum, as well as any unpaid interest and other fees, shall be due and payable. The note is comprised of an initial cash purchase of $335,000 (includes $30,000 of OID and $5,000 for legal fees) (“Initial Note”), a Secured Investor Note of $220,000 (includes $20,000 of OID) (“Secured Investor Note”) and three Investor Notes of $220,000 each (include $20,000 of OID each) (“Investor Notes”). The Secured Investor Note is secured by the lender’s 40% membership interest in a certain LLC. Upon execution of the note, the note holder made an initial cash payment of $300,000 (net of a $30,000 OID and $5,000 for legal fees) to the Company of the total consideration and issued the Secured Investor Note and three Investor Notes to the Company. The Initial Note is convertible, at the option of the lender, to common stock of the Company at any time after the effective date at a price of $0.07 per share, which represents fair value at execution date.
On July 14, 2015, the Company received payment of three Note Receivables of $352,500, that offset three of the Back-End Notes that were issued on May 19, 2015. Proceeds from the Note Receivables of $17,690 were paid directly to legal fees resulting in net cash proceeds of $334,810 received by the Company. These Back-End Note are related to the initial convertible notes that was issued on May 19, 2015 and have the same terms as previously discussed. As a result, these Back-End Notes are now eligible for conversion at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received.
On September 24, 2015, (the “Prior Note Issuance Date”), the Company entered into a Promissory Note with a Lender whereby the Lender loaned the Company a principal amount of $1,200,000 in exchange for the issuance of a Promissory Note (the “Prior Promissory Note”). On October 1, 2015, the Company received cash of $1,150,000 ($1,200,000 less $50,000 of legal fees) for the Prior Promissory Note. As described under “Purchase Agreement” below, the Prior Promissory was deemed cancelled in connection with the October 2015 Financing (as described below) and the principal amount of the Prior Promissory Note was rolled into and included in the Principal Amount in the October 2015 Financing.
78
On October 14, 2015 and October 15, 2015, the Company received payment of six Note Receivables of $430,000 that offset the remaining six of the Back-End Notes that were issued on May 19, 2015. Proceeds from the Note Receivables of $22,265 were paid directly to legal fees resulting in net cash proceeds of $407,735 received by the Company. These Back-End Note are related to the initial convertible notes that was issued on May 19, 2015 and have the same terms as previously discussed. As a result, these Back-End Notes are now eligible for conversion at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received.
Purchase Agreement
On October 28, 2015 (the “Closing Date”), the Company entered into a securities purchase agreement dated as of the Closing Date (the “Purchase Agreement”) with Delafield Investments Limited (the “Purchaser”). The Purchase Agreement provides that, upon the terms and subject to the conditions set forth therein, the Purchaser will invest $4,000,000 (“Investment Amount”) in exchange for a Convertible Debenture (the “Debenture”) in the principal amount of $4,400,000 and warrants to purchase an aggregate of 26,190,476 shares of the Company’s common stock, par value $0.001 per share, for an exercise price of $0.60 per share for a period of four (4) years from the Closing Date (the “Warrant”). Under the terms of the Purchase Agreement, the Purchaser agreed to deliver the Prior Promissory Note and the parties further agreed that the Prior Promissory Note was deemed cancelled and the amount of the Prior Promissory Note is included in the Investment Amount under the Purchase Agreement.
On March 11, 2016, the Company entered into an Addendum with the Purchaser pursuant to which the Company and the Purchaser agreed to certain new terms with respect to the transactions contemplated by Purchase Agreement (the “Addendum”). The Addendum did not modify the Principal Amount.
Due to the satisfaction of certain conditions set forth in the Debenture, the Company received a $50,000 reduction of the principal amount of the October 2015 Financing such that the current aggregate principal amount is $4,350,000 as of the date of this Registration Statement.
Pursuant to the Purchase Agreement, as modified by the Addendum, on the Closing Date, the Company issued the Debenture and Warrant to the Purchaser. From the Prior Note Issuance Date through the date of this Registration Statement, the Company received an aggregate of $3,625,000 pursuant to the Purchase Agreement and Debenture, which the Company has used, and will use, for working capital and to pay off outstanding debt. The Purchase Agreement is described in more detail above in the section titled “Magna Financing.”
The Company claimed an exemption from the registration requirements of the Securities Act of 1933, as amended (the “Act”) for these securities pursuant to Section 4(2) of the Act and/or Rule 506 of Regulation D promulgated thereunder since, among other things, the transaction did not involve a public offering, the Investor was an “accredited investor” and/or qualified institutional buyers, the Investor had access to information about the Company and its investment, the Investor took the securities for investment and not resale, and we took appropriate measures to restrict the transfer of the securities
Item 16. Exhibits and Financial Statement Schedules.
| Exhibit Number |
Description | |
| 4.1 | Promissory Note issued to Southridge Partners II, L.P., dated July 17, 2014, incorporated by reference to Exhibit 4.11 to the Annual Report on Form 10-K filed on October 14, 2014. | |
| 4.2 | Promissory Note with Lender dated September 24, 2015, incorporated by reference to Exhibit 4.1 to the Current Report on Form 8-K filed on September 29, 2015. | |
| 4.3 | Debenture dated October 28, 2015, incorporated by reference to Exhibit 4.1 to the Current Report on Form 8-K filed on November 3, 2015. | |
| 4.4 | Warrant dated October 28, 2015, incorporated by reference to Exhibit 4.2 to the Current Report on Form 8-K filed on November 3, 2015. | |
79
| 5.1 | Opinion of Szaferman, Lakind, Blumstein & Blader, P.C. regarding the legality of the securities being registered* |
| 10.1 | Settlement Agreement and Stipulation between the Company and Tarpon dated July 18, 2014, incorporated by reference to Exhibit 10.4 to the Current Report on Form 8-K filed on September 23, 2014. | |
| 10.2 | Order Granting Approval of Settlement Agreement and Stipulation between the Company and Tarpon dated September 9, 2014, incorporated by reference to Exhibit 10.5 to the Current Report on Form 8-K filed on September 23, 2014. | |
| 10.3 | Form of Equity Purchase Agreement between the Company and Southridge, incorporated by reference to Exhibit 10.6 to the Current Report on Form 8-K filed on September 23, 2014. | |
| 10.4 | Form of Registration Rights Agreement between Company and Southridge, incorporated by reference to Exhibit 10.7 to the Current Report on Form 8-K filed on September 23, 2014. | |
| 10.5 | Security Agreement dated September 24, 2015, incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed on September 29, 2015. | |
| 10.6 | Securities Purchase Agreement dated October 28, 2015, incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed on November 3, 2015. | |
| 10.7 | Registration Rights Agreement dated October 28, 2015, incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed on November 3, 2015. | |
| 10.8 | Security Agreement dated October 28, 2015, incorporated by reference to Exhibit 10.3 to the Current Report on Form 8-K filed on November 3, 2015. | |
| 10.9 | Addendum, dated March 11, 2016, incorporated by reference to Exhibit 10.4 to the Current Report on Form 8-K filed on March 11, 2016 | |
| 10.10 | Employment Agreement entered into as of February 25, 2015 by and between James Nathanielsz and Propanc Health Group Corporation | |
| 10.11 | Director Agreement entered into as of February 25, 2015 by and between Julian Kenyon Nathanielsz and Propanc Health Group Corporation | |
| 10.12 | Form of Scientific Advisory Board Member Agreement | |
| 23.1 | Consent of Salberg & Company, P.A. | |
| 101.INS | XBRL Instance Document | |
| 101.SCH | XBRL Schema Document | |
| 101.CAL | XBRL Calculation Linkbase Document | |
| 101.LAB | XBRL Label Linkbase Document | |
| 101.PRE | XBRL Presentation Linkbase Document | |
| 101.DEF | XBRL Definition Linkbase Document |
*To be filed by amendment.
80
Item 17. Undertakings.
(A) The undersigned Registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
i. To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
ii. To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the "Calculation of Registration Fee" table in the effective registration statement.
iii. To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(5) Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
81
82
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONSOLIDATED BALANCE SHEETS
| December 31, 2015 | June 30, 2015 | |||||||
| (unaudited) | ||||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | $ | 1,168,118 | $ | 107,627 | ||||
| GST tax receivable | 6,808 | 11,647 | ||||||
| Prepaid expenses and other current assets | 327,785 | 502,616 | ||||||
| TOTAL CURRENT ASSETS | 1,502,711 | 621,890 | ||||||
| Security deposit | 1,606 | 1,684 | ||||||
| Property and equipment, net | 3,671 | 3,494 | ||||||
| TOTAL ASSETS | $ | 1,507,988 | $ | 627,068 | ||||
| LIABILITIES AND STOCKHOLDERS' DEFICIT | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable | $ | 107,134 | $ | 236,466 | ||||
| Accrued expenses and other payables | 43,505 | 386,311 | ||||||
| Convertible notes and related accrued interest, net | 1,949,519 | 1,794,375 | ||||||
| Loans payable | 2,189 | 27,558 | ||||||
| Embedded conversion option liabilities | 2,511,405 | 780,281 | ||||||
| Warrant derivative liability | 82,086 | 269,648 | ||||||
| Due to directors - related parties | 33,471 | 35,108 | ||||||
| Loans from directors and officer - related parties | 54,005 | 79,416 | ||||||
| Employee benefit liability | 80,538 | 71,421 | ||||||
| TOTAL CURRENT LIABILITIES | 4,863,852 | 3,680,584 | ||||||
| Commitments and Contingencies (See Note 7) | - | - | ||||||
| STOCKHOLDERS' DEFICIT: | ||||||||
| Series A preferred stock, $0.01 par value; 10,000,000 shares authorized; 500,000 and 500,000 shares issued and outstanding as of December 31, 2015 and June 30, 2015, respectively | 5,000 | 5,000 | ||||||
| Series B preferred stock, $0.01 par value; 5 shares authorized; 1 and 1 share issued and outstanding as of December 31, 2015 and June 30, 2015, respectively | - | - | ||||||
| Common stock, $0.001 par value; 2,000,000,000 shares authorized; 432,719,886 and 347,442,013 shares issued and outstanding as of December 31, 2015 and June 30, 2015, respectively | 432,720 | 347,442 | ||||||
| Additional paid-in capital | 21,360,201 | 17,458,745 | ||||||
| Accumulated other comprehensive income (loss) | 212,847 | 100,968 | ||||||
| Accumulated deficit | (25,366,632 | ) | (20,965,671 | ) | ||||
| TOTAL STOCKHOLDERS' DEFICIT | (3,355,864 | ) | (3,053,516 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT | $ | 1,507,988 | $ | 627,068 | ||||
The accompanying unaudited condensed notes are an integral part of these unaudited consolidated financial statements.
83
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF OPERATIONS AND OTHER COMPREHENSIVE INCOME (LOSS)
(unaudited)
| Three Months Ended December 31, | Six Months Ended December 31, | |||||||||||||||
| 2015 | 2014 | 2015 | 2014 | |||||||||||||
| REVENUE | ||||||||||||||||
| Revenue | $ | - | $ | - | $ | - | $ | - | ||||||||
| OPERATING EXPENSES | ||||||||||||||||
| Administration expenses | 996,129 | 244,431 | 1,845,108 | 475,913 | ||||||||||||
| Occupancy expenses | 4,889 | 2,567 | 9,827 | 5,344 | ||||||||||||
| Research and development | 142,803 | 2,443 | 296,277 | 6,322 | ||||||||||||
| TOTAL OPERATING EXPENSES | 1,143,821 | 249,441 | 2,151,212 | 487,579 | ||||||||||||
| LOSS FROM OPERATIONS | (1,143,821 | ) | (249,441 | ) | (2,151,212 | ) | (487,579 | ) | ||||||||
| OTHER INCOME (EXPENSE) | ||||||||||||||||
| Interest expense | (1,155,645 | ) | (100,176 | ) | (1,574,289 | ) | (648,655 | ) | ||||||||
| Interest income | - | - | 2,027 | 3 | ||||||||||||
| Other expense | - | - | - | (50,002 | ) | |||||||||||
| Change in fair value of derivative liabilities | (1,347,743 | ) | 8,206 | (551,890 | ) | 122,742 | ||||||||||
| Gain (loss) on debt settlements, net | (58,893 | ) | 71,147 | (58,893 | ) | 34,884 | ||||||||||
| Foreign currency transaction gain (loss) | 72,035 | (157,655 | ) | (138,704 | ) | (182,612 | ) | |||||||||
| TOTAL OTHER INCOME (EXPENSE) | (2,490,246 | ) | (178,478 | ) | (2,321,749 | ) | (723,640 | ) | ||||||||
| LOSS BEFORE INCOME TAXES | (3,634,067 | ) | (427,919 | ) | (4,472,961 | ) | (1,211,219 | ) | ||||||||
| INCOME TAX BENEFIT | 72,000 | 82,440 | 72,000 | 82,440 | ||||||||||||
| NET INCOME (LOSS) | (3,562,067 | ) | (345,479 | ) | (4,400,961 | ) | (1,128,779 | ) | ||||||||
| OTHER COMPREHENSIVE INCOME (LOSS) | ||||||||||||||||
| Foreign currency translation gain (loss) | (146,551 | ) | 103,320 | 111,879 | 218,137 | |||||||||||
| TOTAL OTHER COMPREHENSIVE INCOME (LOSS) | $ | (3,708,618 | ) | $ | (242,159 | ) | $ | (4,289,082 | ) | $ | (910,642 | ) | ||||
| BASIC AND DILUTED NET LOSS PER SHARE | $ | (0.01 | ) | $ | (0.00 | ) | $ | (0.01 | ) | $ | (0.01 | ) | ||||
| BASIC AND DILUTED WEIGHTED AVERAGE SHARES OUTSTANDING | 399,822,354 | 95,007,061 | 375,025,485 | 85,827,403 | ||||||||||||
The accompanying unaudited condensed notes are an integral part of these unaudited consolidated financial statements.
84
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF CASH FLOWS
(unaudited)
| Six Months Ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (4,400,961 | ) | $ | (1,128,779 | ) | ||
| Adjustments to Reconcile Net loss to Net Cash Used in Operating Activities: | ||||||||
| Issuance and amortization of common stock for services | 1,122,322 | 112,268 | ||||||
| Issuance of preferred stock for services | - | 1,067 | ||||||
| (Gain) loss on settlement | 58,893 | 355,000 | ||||||
| Settlement fees paid in the form of debt | - | 125,000 | ||||||
| Other | - | 100 | ||||||
| Foreign currency transaction loss | (20,509 | ) | - | |||||
| Depreciation expense | 340 | - | ||||||
| Amortization of debt discount | 1,224,235 | 117,231 | ||||||
| Change in fair value of derivative liabilities | 551,890 | (122,742 | ) | |||||
| Accretion of put premium | 755,927 | 165,428 | ||||||
| Changes in Assets and Liabilities: | ||||||||
| GST receivable | 4,296 | (1,226 | ) | |||||
| Prepaid expenses and other assets | (343,259 | ) | - | |||||
| Accounts payable | (118,305 | ) | 46,849 | |||||
| Employee benefit liability | 12,447 | 3,915 | ||||||
| Accrued expenses | (324,789 | ) | 46,585 | |||||
| Accrued interest | (10,005 | ) | 7,308 | |||||
| Other | (744 | ) | - | |||||
| NET CASH USED IN OPERATING ACTIVITIES | (1,488,222 | ) | (271,996 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Purchase of equipment | (676 | ) | - | |||||
| NET CASH USED IN INVESTING ACTIVITIES | (676 | ) | - | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Loan repayments to principal stockholder | (21,500 | ) | (20,000 | ) | ||||
| Loan repayments | (23,852 | ) | - | |||||
| Proceeds from convertible promissory notes | 2,977,500 | 90,000 | ||||||
| Repayments of convertible promissory notes | (463,976 | ) | - | |||||
| Proceeds from issuance of common stock for cash | - | 29,000 | ||||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 2,468,172 | 99,000 | ||||||
| Effect of exchange rate changes on cash | 81,217 | 99,785 | ||||||
| NET INCREASE (DECREASE) IN CASH | 1,060,491 | (73,211 | ) | |||||
| CASH AT BEGINNING OF PERIOD | 107,627 | 87,799 | ||||||
| CASH AT END OF PERIOD | $ | 1,168,118 | $ | 14,588 | ||||
| Supplemental Disclosure of Cash Flow Information | ||||||||
| Cash paid during the period: | ||||||||
| Interest | $ | - | $ | - | ||||
| Income Tax | $ | - | $ | - | ||||
| Supplemental Disclosure of Non-Cash Investing and Financing Activities | ||||||||
| Prepaid common stock issued for services | $ | 187,532 | $ | 134,373 | ||||
| Reduction of put premium related to conversions of convertible note | $ | 636,348 | $ | 37,032 | ||||
| Conversion of convertible notes and accrued interest to common stock | $ | 1,762,430 | $ | 177,567 | ||||
| Discounts related to warrants issued with convertible debenture | $ | 1,619,075 | $ | - | ||||
| Discounts related to lender costs | $ | - | $ | 33,500 | ||||
| Discounts related to derivative liability | $ | 1,005,925 | $ | - | ||||
| Conversion of loan payable to common stock | $ | - | $ | 66,389 | ||||
The accompanying unaudited condensed notes are an integral part of these unaudited consolidated financial statements.
85
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
NOTE 1 – NATURE OF OPERATIONS AND SUMMARY OF SIGNIFICANT ACCOUNTING AND REPORTING POLICIES
Nature of Operations
Propanc PTY LTD was incorporated in Melbourne, Victoria Australia on October 15, 2007, and is based in Richmond, Victoria Australia. Since inception, substantially all of the efforts of the Company have been the development of new cancer treatments targeting high risk patients who need a follow up, nontoxic, long term therapy which prevents the cancer from returning and spreading. The Company anticipates establishing global markets for its technologies.
On November 23, 2010, Propanc Health Group Corporation ("the Company", "we", "us", "our") was incorporated in the state of Delaware. In January 2011, to reorganize the Company, Propanc Health Group Corporation acquired all of the outstanding shares of Propanc PTY LTD on a one-for-one basis making it a wholly-owned subsidiary.
Basis of Presentation
The interim unaudited consolidated financial statements included herein have been prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”), and pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”). In the opinion of the Company’s management, all adjustments (consisting of normal recurring adjustments and reclassifications and non-recurring adjustments) necessary to present fairly our results of operations for the three and six months ended December 31, 2015 and 2014 and cash flows for the six months ended December 31, 2015 and 2014 and our financial position as of December 31, 2015 have been made. The results of operations for such interim periods are not necessarily indicative of the operating results to be expected for the full year.
Certain information and disclosures normally included in the notes to the annual audited consolidated financial statements have been condensed or omitted from these interim unaudited consolidated financial statements. Accordingly, these interim unaudited consolidated financial statements should be read in conjunction with the audited consolidated financial statements and notes thereto for the fiscal year ended June 30, 2015. The June 30, 2015 balance sheet is derived from those statements.
Principals of Consolidation
The unaudited consolidated financial statements include the accounts of Propanc Health Group Corporation and its wholly-owned subsidiary, Propanc PTY LTD. All significant inter-company balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates. Significant estimates in the accompanying unaudited consolidated financial statements include the estimates of useful lives for depreciation, valuation of derivatives, valuation of beneficial conversion features on convertible debt, allowance for uncollectable receivables, valuation of equity based instruments issued for other than cash, the valuation allowance on deferred tax assets and foreign currency translation due to certain average exchange rates applied in lieu of spot rates on transaction dates.
86
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
Foreign Currency Translation and Other Comprehensive Income (Loss)
The Company’s functional currency is the Australian dollar (AUD). For financial reporting purposes, the Australian dollar has been translated into United States dollars ($) and/or USD as the reporting currency. Assets and liabilities are translated at the exchange rate in effect at the balance sheet date. Revenues and expenses are translated at the average rate of exchange prevailing during the reporting period. Equity transactions are translated at each historical transaction date spot rate. Translation adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’ equity (deficit) as “accumulated other comprehensive income (loss).” Gains and losses resulting from foreign currency transactions are included in the statements of operations and other comprehensive income (loss) as other income (expense). There have been no significant fluctuations in the exchange rate for the conversion of Australian dollars to USD after the balance sheet date.
Other Comprehensive Income (Loss) for all periods presented, includes only foreign currency translation gains (losses).
Changes in Accumulated Other Comprehensive Income (Loss) by Component during the six months ended December 31, 2015 was as follows:
| Foreign Currency Items: | ||||
| Beginning balance, June 30, 2015 | $ | 100,968 | ||
| Foreign currency translation gain | 111,879 | |||
| Ending balance, December 31, 2015 | $ | 212,847 | ||
Fair Value of Financial Instruments and Fair Value Measurements
The Company measures their financial assets and liabilities in accordance with US GAAP. For certain of the Company’s financial instruments, including cash and cash equivalents, accounts and other receivables, accounts payable and accrued expenses and other liabilities, the carrying amounts approximate fair value due to their short maturities. Amounts recorded for loans payable, also approximate fair value because current interest rates available to us for debt with similar terms and maturities are substantially the same.
The Company adopted accounting guidance for fair value measurements of financial assets and liabilities. The adoption did not have a material impact on the Company’s results of operations, financial position or liquidity. This standard defines fair value, provides guidance for measuring fair value and requires certain disclosures. This standard does not require any new fair value measurements, but rather applies to all other accounting pronouncements that require or permit fair value measurements. This guidance does not apply to measurements related to share-based payments. This guidance discusses valuation techniques, such as the market approach (comparable market prices), the income approach (present value of future income or cash flow), and the cost approach (cost to replace the service capacity of an asset or replacement cost). The guidance utilizes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The following is a brief description of those three levels:
Level 1: Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2: Inputs other than quoted prices that are observable, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active.
Level 3: Unobservable inputs in which little or no market data exists, therefore developed using estimates and assumptions developed by us, which reflect those that a market participant would use.
87
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
Cash and Cash Equivalents
Cash and cash equivalents include cash on hand and at banks, short-term deposits with an original maturity of three months or less with financial institutions, and bank overdrafts. Bank overdrafts are reflected as a current liability on the balance sheets. There were no cash equivalents as of December 31, 2015 or June 30, 2015.
Patents
Patent costs are stated at cost and reclassified to intangible assets and amortized on a straight-line basis over the estimated future periods if and once the patent has been granted by a regulatory agency, however, the Company will expense any costs as long as the Company is in the startup stage. Accordingly, as the Company's product was and is not currently approved for market, thus any patent costs incurred from 2013 through 2015 were expensed immediately. Currently, the Company has one International patent pending which was jointly applied for by the Company and another entity.
The Company received grant status, or has been accepted in South Africa, Australia, and New Zealand. In addition, the United States Patent and Trademark Office or USPTO and European Patent Office or EPO have made preliminary indications that key features of the Company’s technology are patentable. The Company is presently working towards securing a patent in each region, covering as many aspects of its technology as possible, whilst also actively seeking protection throughout Eastern Europe, Asia and South America.
Individual countries and regions where the Company is actively seeking patent protection include United States, Canada, Japan, Brazil, China, Mexico, Hong Kong, Singapore, Israel, Chile, Peru, Malaysia, Vietnam, Indonesia, Europe, Russia, India, and South Korea. The patent is now granted, or accepted in South Africa, Australia, and New Zealand.
Impairment of Long-Lived Assets
In accordance with ASC 360-10, Long-lived assets, which include property and equipment and intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of long-lived assets to be held and used is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the assets. Fair value is generally determined using the asset’s expected future discounted cash flows or market value, if readily determinable.
Australian Goods and Services Tax (GST)
Revenues, expenses and balance sheet items are recognized net of the amount of GST except payable and receivable balances which are shown inclusive of GST. The GST incurred is payable on revenues to, and recoverable on purchases from, the Australian Taxation Office.
Cash flows are presented in the statements of cash flow on a gross basis, except for the GST component of investing and financing activities, which are disclosed as operating cash flows.
As of December 31, 2015 and June 30, 2015 the Company was owed $6,808 and $11,647 from the Australian Taxation Office. These amounts were fully collected subsequent to the balance sheet reporting dates.
88
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
Derivative Instruments
ASC Topic 815, Derivatives and Hedging (“ASC Topic 815”), establishes accounting and reporting standards for derivative instruments and for hedging activities by requiring that all derivatives be recognized in the balance sheet and measured at fair value. Gains or losses resulting from changes in the fair value of derivatives are recognized in earnings or recorded in other comprehensive income (loss) depending on the purpose of the derivatives and whether they qualify and have been designated for hedge accounting treatment. The Company does not have any derivative instruments for which it has applied hedge accounting treatment.
Convertible Notes With Variable Conversion Options
The Company has entered into convertible notes, some of which contain variable conversion options, whereby the outstanding principal and accrued interest may be converted, by the holder, into common shares at a fixed discount to the price of the common stock at the time of conversion. The Company treats these convertible notes as stock settled debt under ASC 480 and measures the fair value of the notes at the time of issuance, which is the result of the share price discount at the time of conversion, and records the put premium as accretion to interest expense to the date of first conversion.
Income Taxes
The Company is governed by Australia and United States income tax laws, which are administered by the Australian Taxation Office and the United States Internal Revenue Service, respectively. The Company follows FASB ASC 740 when accounting for income taxes, which requires an asset and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed annually for temporary differences between the financial statements and tax bases of assets and liabilities that will result in taxable or deductible amounts in the future based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Income tax expense is the tax payable or refundable for the period plus or minus the change during the period in deferred tax assets and liabilities.
The Company adopted provisions of ASC 740, Sections 25 through 60, “Accounting for Uncertainty in Income Taxes." These sections provide detailed guidance for the financial statement recognition, measurement and disclosure of uncertain tax positions recognized in the financial statements. Tax positions must meet a “more-likely-than-not” recognition threshold at the effective date to be recognized upon the adoption of ASC 740 and in subsequent periods.
Research and Development Costs and Tax Credits
In accordance with ASC 730-10, research and development costs are expensed when incurred. Total research and development costs for the six months ended December 31, 2015 and 2014 were $296,277 and $6,322 respectively.
The Company may apply for research and development tax concessions with the Australian Taxation Office on an annual basis. Although the amount is possible to estimate at year end, the Australian Taxation Office may reject or materially alter the claim amount. Accordingly, the Company does not recognize the benefit of the claim amount until cash receipt since collectability is not certain until such time. The tax concession is a refundable credit. If the Company has net income then the Company can receive the credit which reduces its income tax liability. If the Company has net losses then the Company may still receive a cash payment for the credit, however, the Company's net operating loss carryforwards are reduced by the gross equivalent loss that would produce the credit amount when the income tax rate is applied to that gross amount. The concession is recognized as an income tax benefit, in operations, upon receipt.
Stock Based Compensation
The Company records stock based compensation in accordance with ASC section 718, “Stock Compensation” and Staff Accounting Bulletin (SAB) No. 107 (SAB 107) issued by the SEC in March 2005 regarding its interpretation of ASC 718. ASC 718 requires the fair value of all stock-based employee compensation awarded to employees to be recorded as an expense over the related requisite service period. The Company values any employee or non-employee stock based compensation at fair value using the Black-Scholes Option Pricing Model.
The Company accounts for non-employee share-based awards in accordance with the measurement and recognition criteria of ASC 505-50 “Equity-Based Payments to Non-Employees”.
89
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
Revenue Recognition
In accordance with SEC Staff Accounting Bulletin (SAB) No. 104, Revenue Recognition, (codified in ASC 605) the Company recognizes revenue when (i) persuasive evidence of a customer or distributor arrangement exists or acceptance occurs, (ii) a retailer, distributor or wholesaler receives the goods, (iii) the price is fixed or determinable, and (iv) collectability of the sales revenues is reasonably assured. Subject to these criteria, the Company recognizes revenue relating to royalties on product sales in the period in which the sale occurs and the royalty term has begun.
Basic and Diluted Net Loss Per Common Share
Basic net loss per share is computed by dividing the net loss by the weighted average number of common shares outstanding during the period. Diluted net loss per common share is computed by dividing the net loss by the weighted average number of common shares outstanding for the period and, if dilutive, potential common shares outstanding during the period. Potentially dilutive securities consist of the incremental common shares issuable upon exercise of common stock equivalents such as stock options, warrants and convertible debt instruments. Potentially dilutive securities are excluded from the computation if their effect is anti-dilutive. As a result, the basic and diluted per share amounts for all periods presented are identical. For the six months ended December 31 2015, there were 33,569,634 warrants outstanding and seventeen convertible notes payable that are convertible into 409,272,805 common shares respectively which are considered dilutive securities which were excluded from the computation since the effect is anti-dilutive.
Recently Adopted Accounting Pronouncements
Financial Accounting Standards Board, Accounting Standard Updates which are not effective until after December 31, 2015 are not expected to have a significant effect on the Company’s consolidated financial position or results of operations. The Company is evaluating or has implemented the following at December 31, 2015:
In August 2014, the FASB issued ASU 2014-15, “Presentation of Financial Statements – Going Concern (Topic 205-40)”, which requires management to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern for each annual and interim reporting period. If substantial doubt exists, additional disclosure is required. This new standard will be effective for the Company for annual and interim periods beginning after December 15, 2016. Early adoption is permitted. The Company expects to adopt this new standard as of December 31, 2016. The Company does not expect this ASU to have a material impact on its consolidated financial statements.
On May 8, 2015, the FASB issued ASU 2015-08, “Business Combinations (Topic 805) Pushdown Accounting” which conforms the FASB’s guidance on pushdown accounting with the SEC’s guidance. ASU 2015-08 is effective for annual periods beginning after December 15, 2015. As of December 31, 2015, this ASU has not had a material impact on the unaudited consolidated financial statements.
In April 2015, the Financial Accounting Standards Board issued Accounting Standards Update No. 2015-03, "Simplifying the Presentation of Debt Issuance Costs," which changes the presentation of debt issuance costs in financial statements. Under this guidance such costs would be presented as a direct deduction from the related debt liability rather than as an asset. This guidance is effective for interim and annual reporting periods beginning after December 15, 2015. As of December 31, 2015, this ASU has not had a material impact on the unaudited consolidated balances current presentation.
NOTE 2 – GOING CONCERN
The accompanying unaudited consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America, which contemplate continuation of the Company as a going concern. For the six months ended December 31, 2015, the Company had no revenues and had a net loss of $4,400,961 and net cash used in operations of $1,488,222. Additionally, as of December 31, 2015, the Company had a working capital deficit, stockholders' deficit and accumulated deficit of $3,361,141, $3,355,864, and $25,366,632 respectively. These conditions raise substantial doubt about the Company’s ability to continue as a going concern.
90
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
The unaudited consolidated financial statements do not include any adjustments to reflect the possible future effect on the recoverability and classification of assets or the amounts and classifications of liabilities that may result from the outcome of this uncertainty.
Successful completion of the Company’s development program and, ultimately, the attainment of profitable operations are dependent upon future events, including obtaining adequate financing to fulfill its development activities, acceptance of the Company's International patent application and achieving a level of sales adequate to support the Company’s cost structure. However, there can be no assurances that the Company will be able to secure additional equity investment or achieve an adequate sales level.
NOTE 3 – DUE TO DIRECTORS - RELATED PARTIES
Due to directors - related parties represents unsecured advances made by the directors for operating expenses on behalf of the Company such as intellectual property and formation expenses. The expenses were paid for on behalf of the Company are due upon demand. The Company is currently not being charged interest under these advances. The total amount owed these directors at December 31, 2015 and June 30, 2015 is $33,471 and $35,108 respectively.
NOTE 4 – LOANS
Loans from Directors and Officer - Related Parties
Loans from Directors and an Officer at December 31, 2015 and June 30, 2015 were $54,005 and $79,416, respectively. The loans bear no interest and are all past their due date and in default. The Company repaid cash of $21,500 (AUD$29,744) of these loans during the six months ended December 31, 2015.
Other Loans from Unrelated Parties
As of June 30, 2015, other loans from unrelated parties balance was $27,558. During the six months ended December 31, 2015, the Company repaid cash of $23,852 (AUD$33,000) and a foreign currency transaction gain of $1,517 resulting in a balance of $2,189 as of December 31, 2015.
NOTE 5 – CONVERTIBLE NOTES
Convertible notes at December 31, 2015 were as follows:
| Convertible notes and debenture | $ | 3,630,024 | ||
| Unamortized discounts | (2,341,232 | ) | ||
| Accrued interest | 82,802 | |||
| Premium | 577,925 | |||
| Convertible notes, net | $ | 1,949,519 |
On August 6, 2014 (execution date), the Company executed a convertible promissory note in the principal sum of $250,000, with an original issue discount (“OID”) of $25,000. The consideration to be paid to the Lender shall be equal to the consideration actually paid by the Lender plus prorated interest and any other fees that the Company shall be required to pay. The original issue discount shall also be prorated based on the actual consideration received to equal approximately 10% of the consideration received. If the Company repays a consideration payment on or before the first 90 days from the effective date of that payment, the interest rate on that payment of consideration will be 0%. If the Company does not repay a payment on or before the 90 days, the Company will incur a one-time interest charge of 12% on the principal amount of the loan. Upon execution of the note, the note holder made an initial payment of $25,000 (net of a $2,500 OID) to the Company of the total consideration. The maturity date is two years from the date of each payment to the Company, and is the date upon which the principal sum, as well as any unpaid interest and other fees, shall be due and payable. The note is convertible, at the option of the investor, to common stock of the Company at any time after the effective date at the lesser of $0.09 or 60% of the lowest trade price in the 25 trading days prior to the conversion. The Company didn’t repay the consideration payment on or before the first 90 days from the effective date of that payment and therefore incurred a 12% interest charge. No further funding other than the above mentioned $25,000 has been received under the $250,000 note. On December 10, 2015, the Company repaid cash of $90,000 as payment in full of $27,500 of principal and accrued interest of $3,607 resulting in $58,893 of a penalty which was expensed as loss on debt settlement. As of December 31, 2015, this note was paid in full.
91
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
On February 10, 2015, the Company issued a convertible note payable for $45,000 with an OID of $7,500. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is February 10, 2016. The note is convertible at the option of the holder at any time after 180 days at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days prior to the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $36,818 put premium over 180 days from the execution of the convertible note. During the six months ended December 31, 2015, the Company has accreted the remaining $9,409 of the put premium as $27,409 had been accreted at June 30, 2015, resulting in the put premium being fully expensed. During the six months ended December 31, 2015, the Company converted $45,000 of principal and accrued interest of $1,887 into shares of the Company’s common stock (See Note 6). Additionally, this conversion resulted in a $36,818 reduction of the put premium. As of December 31, 2015, this note was fully converted.
On February 17, 2015, the Company issued a second convertible note payable for $45,000 with an OID of $7,500. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is February 17, 2016. The note is convertible at the option of the holder at any time after 180 days at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days prior to the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $36,818 put premium over 180 days from the execution of the convertible note. During the six months ended December 31, 2015, the Company has accreted the remaining $9,409 of the put premium as $27,409 had been accreted at June 30, 2015, resulting in the put premium being fully expensed. During the six months ended December 31, 2015, the Company converted $45,000 of principal and accrued interest of $2,229 into shares of the Company’s common stock (See Note 6). Additionally, this conversion resulted in a $36,818 reduction of the put premium. As of December 31, 2015, this note was fully converted.
On March 12, 2015, the Company issued a third convertible note payable for $170,500 with an OID of $13,000. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is March 12, 2016. The note is convertible at the option of the holder at any time at a rate of 55% of the Company’s common stock for the average of the lowest three trading prices in the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company recognized a $139,500 put premium. During the six months ended December 31, 2015, the Company converted $170,500 of principal and accrued interest of $8,580 into shares of the Company’s common stock (See Note 6). Additionally, this conversion resulted in a $139,500 reduction of the put premium. As of December 31, 2015, this note was fully converted.
On March 20, 2015, the Company issued a fourth convertible note payable for $150,000. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is March 20, 2016. The note is convertible at the option of the holder at any time at a rate of 55% of the lowest trading bid price of the Company’s common stock for the average of the lowest three trading priced in the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company recognized a $122,727 put premium. During the six months ended December 31, 2015, the Company converted $150,000 of principal and accrued interest of $9,436 into shares of the Company’s common stock (See Note 6). Additionally, this conversion resulted in a $122,727 reduction of the put premium. As of December 31, 2015, this note was fully converted.
92
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
In addition to each of the above initial convertible promissory notes (“initial convertible notes”), the Company issued to each lender another convertible promissory note for the same amounts of $45,000, $45,000, $170,500 and $150,000 termed "Back-End Notes". These notes have the same terms as the initial convertible notes. Each Back-End Note shall initially be paid for by an offsetting promissory note issued to the Company by the lender ("Note Receivable") provided that prior to the conversion of the Back-End Notes, the holders must have paid off the Notes Receivable in cash. Each Note Receivable is due eight months from issuance of each initial convertible note, unless the Company does not meet the “current public information” requirement pursuant to Rule 144, in which case both the Back-End Notes and the Notes Receivable may both be cancelled. Each Note Receivable is initially secured by the pledge of the Back-End Notes, but may be exchanged for other collateral with an appraised value of at least the principal amount of the note less the OID, upon Company’s approval following a three (3) day written notice to the Company. The term of the Notes Receivable and the Back-End Notes are one year, upon which the outstanding principal and interest is payable. The amounts funded plus accrued interest under Back-End Notes are convertible into common stock at any time after the requisite Rule 144 holding period (subject to the condition above for the Back-End Notes), at a conversion price equal to 55% of the lowest trading bid price in the ten (10) trading days prior to the conversion. The $45,000, $45,000, $170,500 and $150,000 Back-End Notes were issued as noted below.
In the event the Company redeems the initial convertible notes in full, the Company is required to pay off all principal, interest and any other amounts owing multiplied by i) 130% if prepaid within 60 days of the issuance date; ii) 140% if prepaid 60 but less than 121 days after the issuance date; and (iii) 150% if prepaid 120 but less than 180 days after the issuance date. There shall be no redemption after the 180th day. The Back-End Notes may not be prepaid, except that if the initial convertible notes are redeemed by the Company within six months of their issuance, all obligations of the Company and holders under the Back-End Notes and the Notes Receivable will be deemed satisfied and such notes shall automatically be deemed cancelled and of no further force or effect.
In the event of two specific defaults, which include the maintenance of a minimum trading price and an aggregate dollar trading volume of the Company's common shares, the holders may cancel the Back-End Notes and the related Notes Receivable and otherwise in the event of other defaults as defined in the securities purchase agreement, the amount of principal and accrued interest will become immediately due and payable and may be offset by amounts due to the Company by the holders. Additionally, the Back-End Notes will bear default interest at a rate of 24% per annum, or the highest rate of interest permitted by law.
On February 15, 2015, in connection with a six-month consulting agreement, the Company issued a convertible promissory note for $90,000 as compensation for services to be rendered. The Company agreed to pay 5% interest per annum on the principal amount and the maturity date is August 15, 2015. The note is convertible at the option of the holder at any time after issuance of note at a rate of 60% of the lowest trading price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company fully expensed a $60,000 put premium. During the six months ended December 31, 2015, the Company converted $85,000 of principal into shares of the Company’s common stock (See Note 6). Additionally, this conversion resulted in a $56,667 reduction of the put premium. Accrued interest as of December 31, 2015 was $2,764.
On March 12, 2015, the Company issued a convertible promissory note for $104,000. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is December 16, 2015. The note is convertible at the option of the holder at any time after 180 days at a rate of 58% of the average lowest three trading closing bid prices of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $75,310 put premium over 180 days from the execution of the convertible note. On July 15, 2015, the Company repaid cash of $137,915 as payment in full of $104,000 of principal and accrued interest of $2,872 resulting in $31,043 of a prepayment penalty which was expensed as interest expense. During the six months ended December 31, 2015, the Company has accreted $6,276 of the put premium as $46,441 had been accreted at June 30, 2015 and this repayment resulted in a $22,593 reduction of the remaining put premium. As of December 31, 2015, this note was paid in full.
On March 12, 2015, in connection with a two-year consulting agreement, the Company issued a convertible promissory note for $60,000 as compensation for services to be rendered. The Company agreed to pay 10% interest per annum on the principal amount and the maturity date is March 11, 2017. The note is convertible, at the option of the holder, at any time after the effective date at the lesser of $0.0175 or 75% of the volume weighted average of the lowest three trading closing bid prices of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. This note was bifurcated with the embedded conversion option recorded as a derivative liability at fair value (See Note 10). Accrued interest as of December 31, 2015 was $4,849.
93
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
On April 20, 2015, the Company issued a convertible note payable for $17,500. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is April 20, 2016. The note is convertible at the option of the holder at any time at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company recognized a $14,318 put premium. During the six months ended December 31, 2015, the Company converted $16,500 of principal and accrued interest of $803 into shares of the Company’s common stock (See Note 6). Additionally, this conversion resulted in a $13,500 reduction of the put premium. Accrued interest as of December 31, 2015 was $59.
On April 24, 2015, the Company received payment of the Note Receivable of $45,000, less the OID of $7,500, that offsets the Back-End Note that was issued on February 10, 2015. Proceeds from the Note Receivable of $2,250 were paid directly to legal fees resulting in net cash proceeds of $35,250 received by the Company. This Back-End Note is related to the initial convertible note that was issued on February 10, 2015 and has the same terms as previously discussed. As a result, the Back-End Note is now eligible for conversion at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $36,818 put premium over 180 days from the execution of the convertible note. During the six months ended December 31, 2015, the Company has accreted the remaining $22,909 of the put premium as $13,909 had been accreted at June 30, 2015, resulting in the put premium being fully expensed. During the six months ended December 31, 2015, the Company converted $45,000 of principal and accrued interest of $1,765 into shares of the Company’s common stock (See Note 6). Additionally, this conversion resulted in a $36,818 reduction of the put premium. As of December 31, 2015, this note was fully converted.
On April 24, 2015, the Company received payment of the Note Receivable of $45,000, less the OID of $7,500, that offsets the Back-End Note that was issued on February 17, 2015. Proceeds from the Note Receivable of $2,250 were paid directly to legal fees resulting in net cash proceeds of $35,250 received by the Company. This Back-End Note is related to the initial convertible note that was issued on February 17, 2015 and has the same terms as previously discussed. As a result, the Back-End Note is now eligible for conversion at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $36,818 put premium over 180 days from the execution of the convertible note. During the six months ended December 31, 2015, the Company has accreted the remaining $22,909 of the put premium as $13,909 had been accreted at June 30, 2015, resulting in the put premium being fully expensed. During the six months ended December 31, 2015, the Company converted $1,000 of principal and accrued interest of $46 into shares of the Company’s common stock (See Note 6). Additionally, this conversion resulted in a $818 reduction of the put premium. Accrued interest as of December 31, 2015 was $2,430.
On April 27, 2015, the Company received payment of the Note Receivable of $170,500, less the OID of $13,000, that offsets the Back-End Note that was issued on March 12, 2015. Proceeds from the Note Receivable of $7,500 were paid directly to legal fees resulting in net cash proceeds of $150,000 received by the Company. This Back-End Note is related to the initial convertible note that was issued on March 12, 2015 and has the same terms as previously discussed. As a result, the Back-End Note is now eligible for conversion at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company recognized a $139,500 put premium. During the six months ended December 31, 2015, the Company converted $170,500 of principal and accrued interest of $8,303 into shares of the Company’s common stock (See Note 6). Additionally, this conversion resulted in a $139,500 reduction of the put premium. As of December 31, 2015, this note was fully converted.
94
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
On May 19, 2015, the Company entered into a Securities Purchase Agreement (“SPA”), to issue a series of nine back end convertible notes in the principal sum of $782,500, pursuant to the SPA, the Company issued to the lender nine convertible promissory notes termed "Back-End Notes", in the amounts of $37,500 ("Back-End Note 1"), $37,500 ("Back-End Note 2"), $157,500 ("Back-End Note 3"), $150,000 ("Back-End Note 4"), $17,500 ("Back-End Note 5"), $37,500 ("Back-End Note 6"), $37,500 ("Back-End Note 7"), $157,500 ("Back-End Note 8") and $150,000 ("Back-End Note 9"). These notes have the same terms as the initial convertible notes. Each Back-End Note shall initially be paid for by an offsetting promissory note issued to the Company by the lender ("Note Receivable") provided that prior to the conversion of the Back-End Notes, the holders must have paid off the Notes Receivable in cash. Each Note Receivable is due on May 19, 2016, unless the Company does not meet the “current public information” requirement pursuant to Rule 144, in which case both the Back-End Notes and the Notes Receivable may both be cancelled. Each Note Receivable is initially secured by the pledge of the Back-End Notes, but may be exchanged for other collateral with an appraised value of at least the principal amount of the note less the OID, upon Company’s approval following a three (3) day written notice to the Company. The term of the Notes Receivable and the Back-End Notes are one year, upon which the outstanding principal and interest is payable. The amounts funded plus accrued interest under Back-End Notes are convertible into common stock at any time after the requisite Rule 144 holding period (subject to the condition above for the Back-End Notes), at a conversion price equal to 55% of the lowest trading bid price in the ten (10) trading days prior to the conversion. During the six months ended December 31, 2015, all of the Back-End Notes (an aggregate total principal of $782,500) were issued as noted below.
The Back-End Notes may not be prepaid, except that if the initial convertible notes are redeemed by the Company within six months of their issuance, all obligations of the Company and holders under the Back-End Notes and the Notes Receivable will be deemed satisfied and such notes shall automatically be deemed cancelled and of no further force or effect.
In the event of two specific defaults, which include the maintenance of a minimum trading price and an aggregate dollar trading volume of the Company's common shares, the holders may cancel the Back-End Notes and the related Notes Receivable and otherwise in the event of other defaults as defined in the securities purchase agreement, the amount of principal and accrued interest will become immediately due and payable and may be offset by amounts due to the Company by the holders. Additionally, the Back-End Notes will bear default interest at a rate of 24% per annum, or the highest rate of interest permitted by law.
Since the Back-End Notes are not convertible until the Notes Receivable are paid, and the Notes Receivable and Back-End Notes have a right of setoff, the Notes Receivable and Back-End Notes and related accrued interest receivable and payable have been netted for presentation purposes on the accompanying consolidated balance sheet.
On June 2, 2015, the Company received payment of the Note Receivable of $150,000 that offsets the Back-End Note that was issued on March 20, 2015. Proceeds from the Note Receivable of $7,500 were paid directly to legal fees resulting in net cash proceeds of $142,500 received by the Company. This Back-End Note is related to the initial convertible note that was issued on March 20, 2015 and has the same terms as previously discussed. As a result, the Back-End Note is now eligible for conversion at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company recognized a $122,727 put premium. During the six months ended December 31, 2015, the Company converted $45,000 of principal and accrued interest of $2,140 into shares of the Company’s common stock (See Note 6). Additionally, this conversion resulted in a $36,818 reduction of the put premium. Accrued interest as of December 31, 2015 was $5,740.
On June 4, 2015 (execution date), the Company executed a convertible promissory note in the principal sum of $1,215,000, with an OID of $110,000. The consideration to be paid to the lender shall be equal to the consideration actually paid by the lender plus prorated interest and any other fees that the Company shall be required to pay. The original issue discount shall also be prorated based on the actual consideration received to equal approximately 10% of the consideration received. The Company agreed to pay 10% interest per annum on the principal amount and the maturity date is ten months from the date of each payment to the Company, and is the date upon which the principal sum, as well as any unpaid interest and other fees, shall be due and payable. The note is comprised of an initial cash purchase of $335,000 (includes $30,000 of OID and $5,000 for legal fees) (“Initial Note”), a Secured Investor Note of $220,000 (includes $20,000 of OID) (“Secured Investor Note”) and three Investor Notes of $220,000 each (include $20,000 of OID each) (“Investor Notes”). The Secured Investor Note is secured by the lender’s 40% membership interest in a certain LLC. The Company will accrue 10% interest per annum on the unpaid principal amount of the Secured Investor Note and the three Investor Notes as defined in the agreements. Upon execution of the note, the note holder made an initial cash payment of $300,000 (net of a $30,000 OID and $5,000 for legal fees) to the Company of the total consideration and issued the Secured Investor Note and three Investor Notes to the Company. On July 13, 2015, the Company received payment of the Secured Investor Note of $220,000 less OID of $20,000, that was issued on June 4, 2015. The Company received interest proceeds of $1,997 from the Secured Investor Note resulting in net cash proceeds of $201,997 received by the Company. The Initial Note and the Secured Investor Note are convertible, at the option of the lender, to common stock of the Company at any time after the effective date at a price of $0.07 per share, which represents fair value at execution date. These notes were determined to be derivative instruments due to the variable conversion price of the notes which is initially $0.07 and subject to adjustment if the Company’s market capitalization falls below $3,000,000 at any time. These notes were bifurcated with the embedded conversion option recorded as a derivative liability at fair value (See Note 10). On December 9, 2015, the Company repaid cash of $269,976 as partial payment for this note. The Company is presently in litigation with this lender (See Note 7). Accrued interest as of December 31, 2015 was $29,468. Since the Investor Notes are not convertible until they are paid in cash to the Company and also not for 180 days from the note dates, the remaining principal of this note and the Investor Notes and related accrued interest receivable and payable have been netted for presentation purposes on the accompanying consolidated balance sheet.
95
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
On July 14, 2015, the Company received payment of three Note Receivables of $352,500, that offset three of the Back-End Notes that were issued on May 19, 2015. Proceeds from the Note Receivables of $17,690 were paid directly to legal fees resulting in net cash proceeds of $334,810 received by the Company. These Back-End Note are related to the initial convertible notes that was issued on May 19, 2015 and have the same terms as previously discussed. As a result, these Back-End Notes are now eligible for conversion at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. These convertible notes are treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $288,409 put premium over 180 days from the execution of the convertible notes. During the six months ended December 31, 2015, the Company has accreted $288,409 of the put premium resulting in the put premium being fully expensed. During the six months ended December 31, 2015, the Company converted $20,000 of principal and accrued interest of $690 into shares of the Company’s common stock (See Note 6). Additionally, this conversion resulted in a $16,364 reduction of the put premium. Accrued interest as of December 31, 2015 was $12,308.
On October 1, 2015, the Company received cash of $1,150,000 ($1,200,000 less $50,000 of legal fees) for the Promissory Note issued on September 24, 2015. On September 24, 2015, (the “Issuance Date”), the Company entered into a Promissory Note with a Lender whereby the Lender loaned the Company $1,200,000 in exchange for the issuance of a Promissory Note (the “Promissory Note”). The Company issued the Promissory Note with a principal amount of $1,200,000 to the Lender. The Promissory Note has a maturity date of the earlier of: (i) the date on which the Company closes a subsequent equity offering in an amount greater than the principal amount of the Promissory Note; or (ii) June 24, 2016. On its face, the Promissory Note does not accrue any interest. In the event that the Lender does not proceed with a subsequent financing, beginning on the 46th day following the Issuance Date, the Note will have a one-time interest adjustment of $180,000 on the outstanding principal of the Promissory Note. Additionally, if the Lender does not wish to proceed with a subsequent financing, the Promissory Note will also be convertible into common stock at the lower of (i) $0.0346; or (ii) a twenty percent (20%) discount to the average of the two lowest closing prices of the common stock in the five trading days prior to the date of conversion. In connection with the Promissory Note, the Company entered into a Security Agreement dated September 24, 2015 with the Lender whereby the Company agreed to grant to Lender an unconditional and continuing, first priority security interest in all of the assets and property of the Company to secure the prompt payment, performance and discharge in full of all of Company’s obligations under the Promissory Note, provided, however that in the event the Lender does not proceed with a subsequent financing, any and all security interests shall be removed. On October 28, 2015, the Lender proceeded with a subsequent financing. See below as this note was cancelled on October 28, 2015.
On October 14, 2015 and October 15, 2015, the Company received payment of six Note Receivables of $430,000, that offset the remaining six of the Back-End Notes that were issued on May 19, 2015. Proceeds from the Note Receivables of $22,265 were paid directly to legal fees resulting in net cash proceeds of $407,735 received by the Company. These Back-End Note are related to the initial convertible notes that was issued on May 19, 2015 and have the same terms as previously discussed. As a result, these Back-End Notes are now eligible for conversion at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. These convertible notes are treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $351,818 put premium over 180 days from the execution of the convertible notes. Through December 31, 2015, the Company has accreted $179,818 of the put premium. Accrued interest as of December 31, 2015 was $7,339.
96
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
On October 28, 2015 (the “Closing Date”), the Company entered into a securities purchase agreement dated as of the Closing Date (the “Purchase Agreement”) with a third party purchaser (the “Purchaser”). The Purchase Agreement provides that, upon the terms and subject to the conditions set forth therein, the Purchaser will invest $4,000,000 (“Investment Amount”) in exchange for a Convertible Debenture (the “Debenture”) in the principal amount of $4,400,000 (the “Principal Amount”) and warrants to purchase an aggregate of 26,190,476 shares of the Company’s common stock, par value $0.001 per share, for an exercise price of $0.60 per share for a period of four (4) years from the Closing Date (the “Warrants”). Pursuant to the Purchase Agreement, on the Closing Date, the Company issued the Debenture and Warrant to the Purchaser.
Under the terms of the Purchase Agreement, the Purchaser agreed to deliver the Promissory Note entered into by the Company and Purchaser on September 24, 2015 with a principal amount of $1,200,000 (the “Prior Note”) (as noted above). The parties further agreed that the Prior Note was deemed cancelled upon the delivery by the Purchaser to the Company and the amount of the Prior Note is included in the Investment Amount under the Purchase Agreement.
Under the terms of the Purchase Agreement and Debenture, $2,800,000 of the Investment Amount will be deposited into a deposit control account and such amount will remain in the deposit control account pending the achievement of certain milestones by the Company and the satisfaction of certain equity conditions set forth in the Debenture. Additionally, under the Debenture, the Principal Amount will be reduced by $25,000 if the Company files a registration statement with the SEC within 30 days following the Closing Date. The Principal Amount will be reduced by an additional $25,000 if the registration statement is deemed effective within 100 days after the Closing Date. On November 23, 2015, the Company filed a registration statement with the SEC and on December 10, 2015, the registration statement was deemed effective. The Principal Amount will be reduced by $50,000 after the final deposit is released.
The Purchase Agreement contains customary representations, warranties and covenants by, among and for the benefit of the parties. The Company also agreed to pay up to $50,000 of reasonable attorneys’ fees and expenses incurred by the Purchaser in connection with the transaction. The Purchase Agreement also provides for indemnification of the Purchaser and its affiliates in the event that the Purchaser incurs losses, liabilities, obligations, claims, contingencies, damages, costs and expenses related to a breach by the Company of any of its representations, warranties or covenants under the Purchase Agreement.
The Debenture has a 10% original issue discount and matures on October 28, 2016. The Principal Amount of the Debenture accrues interest at the rate of 5% per annum, payable quarterly in cash (or if certain conditions are met, in stock at the Company’s option) on January 1, April 1, July 1 and October 1. The Debenture is convertible at any time, in whole or in part, at the Purchaser’s option into shares of the Company’s common stock, par value $0.001 per share (the “Common Stock”), at a conversion price equal to $0.042, which is the volume weighted average price of the Company’s Common Stock five days prior to the execution of the Debenture (subject to adjustment) (the “Conversion Price”). At any time after the effective date of the registration statement, the Purchaser has the opportunity to convert up to an aggregate of $2,090,000 of the Debenture, at one or more conversion dates, into shares of Common Stock at a conversion price equal to the VWAP of the Common Stock over the five (5) trading days prior to such Effective Date. The Purchaser option to convert at such a conversion price expires when the Purchaser converts an aggregate of $2,090,000 of the Debenture using such conversion price. If the volume weighted average price of the Company Common Stock on any trading day is less than the Conversion Price, the Purchaser may convert at a price per share equal to a twenty percent (20%) discount to the average of the two lowest closing prices during the five trading days prior to the date of conversion. At no time will the Purchaser be entitled to convert any portion of the Debenture to the extent that after such conversion, the Purchaser (together with its affiliates) would beneficially own more than 4.99% of the outstanding shares of Common Stock as of such date. During the six months ended December 31, 2015, the Company withdrew a principal amount of $1,225,000 from the deposit control account, of which $269,976 was paid directly as partial payment of a note dated June 4, 2015 and $33,437 were paid directly to legal fees resulting in net cash proceeds of $921,587 received by the Company. An aggregate total of $2,090,000 of these notes were bifurcated with the embedded conversion option recorded as a derivative liability at fair value (See Note 10). The remaining principal of $335,000 did not have a beneficial conversion feature value since its conversion price was higher than the fair value of the Company’s common stock. Accrued interest as of December 31, 2015 was $17,844.
97
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
The Debenture includes customary event of default provisions, and provides for a default interest rate of 18%. Upon the occurrence of an event of default, the Purchaser may convert the Debenture into shares of Common Stock at a price per share equal to a thirty percent (30%) discount to the average volume weighted average price of the shares for the three trading days prior to conversion.
Subject to the conditions set forth in the Debenture, the Company has the right at any time to redeem some or all of the total outstanding amount then remaining under the Debenture in cash at a price equal to 125% of the total amount of the Debenture outstanding on the twentieth (20th) trading date following the date the Company delivers notice of such redemption to the Purchaser.
The Warrants are exercisable in whole or in part, at an initial exercise price per share of $0.60, subject to adjustment. The exercise price and number of shares of the Company’s common stock issuable under the Warrants (the “Warrant Shares”) are subject to adjustments for stock dividends, splits, combinations, subsequent rights offerings and pro rata distributions. Any adjustment to the exercise price shall similarly cause the number of warrant shares to be adjusted so that the total value of the Warrants may increase. In the event that the Warrant Shares are not included in an effective registration statement, the Warrants may be exercised on a cashless basis.
In connection with the execution of the Purchase Agreement, on the Closing Date, the Company and the Purchaser also entered into a registration rights agreement dated as of the Closing Date (the “Registration Rights Agreement”). Pursuant to the Registration Rights Agreement, the Company has agreed to file an initial registration statement (“Registration Statement”) with the SEC to register the resale of the Common Stock into which the Debenture may be converted or the Warrant may be exercised, within 30 days following the Closing Date. The Registration Statement must also be declared effective by the 100th calendar day after the Closing Date, subject to a 20-day extension as requested by the Company and consented to by the Purchaser. On November 23, 2015, the Company filed a registration statement with the SEC and on December 10, 2015, the registration statement was deemed effective.
If at any time all of the shares of Common Stock underlying the Debenture or the Warrant are not covered by the initial Registration Statement, the Company has agreed to file with the SEC one or more additional Registration Statements so as to cover all of the shares of Common Stock underlying the Debenture or the Warrant not covered by such initial Registration Statement, in each case, as soon as practicable, but in no event later than the applicable filing deadline for such additional Registration Statements as provided in the Registration Rights Agreement.
In connection with the Purchase Agreement, the Company entered into a Security Agreement dated as of even date therewith with the Purchaser whereby the Company agreed to grant to Purchaser an unconditional and continuing, first priority security interest in all of the assets and property of the Company to secure the prompt payment, performance and discharge in full of all of Company’s obligations under the Debentures, Warrants and the other transaction documents until ten days following the such time as the Registration Statement is declared effective by the SEC and the equity conditions set forth in the Debenture are met.
The Company recorded $2,887,500 and $63,500 of debt discounts for fees paid to lenders related to the above note issuances during the six months ended December 31, 2015 and 2014 respectively. The debt discounts are being amortized over the term of the debt. Amortization of the debt discounts for the six months ended December 31, 2015 and 2014 was $961,735 and $23,970 respectively.
NOTE 6 – STOCKHOLDERS’ DEFICIT
Preferred Stock:
The total number of preferred shares authorized and that may be issued by the Company is 10,000,000 preferred shares with a par value of $0.01. These preferred shares have no rights to dividends, profit sharing or liquidation preferences.
98
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
Of the total preferred shares authorized, pursuant to Certificate of Designation filed on December 9, 2014, 500,000 have been designated as Series A preferred stock, with a par value of $0.01 (“Series A Preferred Stock”). On December 9, 2014, the Company issued 500,000 shares of Series A Preferred Stock to its CEO in consideration for services rendered to the Company, including for and as an incentive to continue to assist and provide services to the Company. The shares were valued at $0.00213 per share for a total value of $1,067 based on the average sale price per share of the 8,161,000 shares of common stock sold during the three months ended December 31, 2014.
Of the total preferred shares authorized, pursuant to Certificate of Designation filed on June 16, 2015, up to five (5) shares have been designated as Series B preferred stock, with a par value of $0.01 (“Series B Preferred Stock”). Each holder of outstanding shares of Series B Preferred Stock shall be entitled to voting power equivalent of the number of votes equal to the total number of Company’ common stock outstanding as of the record date for the determination of stockholders entitled to vote at each meeting of stockholders of the Company and entitled to vote on all matters submitted or required to be submitted to a vote of the stockholders of the Company. On June 16, 2015, the Company issued 1 share of Series B Preferred Stock to its CEO in consideration for services rendered to the Company, including for and as an incentive to continue to assist and provide services to the Company. The share was valued at $0.1165 per share for a total value of $0.12 based on the closing price of the stock on that date. This value represents the economic rights of the share as the value of voting rights, which represent control rights, are not objectively measureable.
Common Stock:
On November 12, 2014, the Company filed an amendment to the Company’s Certificate of Incorporation with the Secretary of State of the State of Delaware, to increase the Company’s authorized common stock from one hundred million (100,000,000) shares of common stock, par value $0.001 per share, to ten billion (10,000,000,000) shares of common stock, par value $0.001 per share. On July 10, 2015, the Company filed an amendment to the Company’s Certificate of Incorporation with the Secretary of State of the State of Delaware, to decrease the Company’s authorized common stock from ten billion (10,000,000,000) shares of common stock, par value $0.001 per share, to two billion (2,000,000,000) shares of common stock, par value $0.001 per share.
Shares issued for services
On June 4, 2015, the Company entered into an agreement with a consultant to provide services over a six month period in exchange for 500,000 shares of common stock. The Company valued the 500,000 shares based on the market price on the agreement date of $0.0706 and will recognize $35,300 of consulting expense through the term of the agreement. On July 2, 2015 the Company issued the 500,000 shares related to this agreement. The Company has recorded $30,285 of consulting expense for the six months ended December 31, 2015.
On August 26, 2015, the Company issued 560,000 shares of common stock to a consultant as compensation for a six month period consulting service. The Company valued the 560,000 shares based on the market price on the issuance date of $0.04. The Company has recorded $15,461 of consulting expense for the six months ended December 31, 2015.
On September 8, 2015, the Company issued 600,000 shares of common stock to a member of the Company’s Scientific Advisory Board. The Company valued the 600,000 shares based on the market price on the issuance date of $0.0369 and immediately expensed the $22,140.
Additionally, during the six months ended December 31, 2015, the Company recognized $407,581 of consulting expense for prepaid services related to shares issued in fiscal 2015.
On July 24, 2015, the Company entered into an agreement with a consultant to provide services over a six month period. The Company agreed to issue the consultant 8,000,000 shares of common stock. The Company valued the 8,000,000 shares based on the market price on the agreement date of $0.0435 and is recognizing $348,000 of consulting expense through the term of the agreement. On October 8, 2015, the Company issued the 8,000,000 shares related to this agreement. The Company has recorded $299,246 of consulting expense for the six months ended December 31, 2015 related to this agreement.
99
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
On October 1, 2015, the Company entered into an agreement with a consultant to provide services over a one year period. The Company agreed to issue the consultant 1,500,000 shares of common stock and an additional 1,500,000 shares of common stock on April 1, 2016 unless the Company terminates the agreement. The Company valued the 1,500,000 shares based on the market price on the agreement date of $0.031 and is recognizing $46,500 of consulting expense over the one year term of the agreement. The Company has recorded $11,720 of consulting expense for the six months ended December 31, 2015 related to this agreement. On October 1, 2015, the Company issued 1,100,000 and 400,000 shares of common stock to consultants related to this agreement.
On October 16, 2015, the Company issued 4,000,000 shares of common stock to a consultant. The Company valued the 4,000,000 shares based on the market price on the issuance date of $0.0415 and is recognizing $166,000 of consulting expense over the six month term of the agreement. The Company has recorded $68,940 of consulting expense for the six months ended December 31, 2015 related to this agreement.
On December 30, 2015, the Company entered into an agreement with a consultant to provide services over a nine month period. The Company agreed to issue the consultant 1,000,000 shares of common stock. The Company valued the 1,000,000 shares based on the market price on the agreement date of $0.0260 and is recognizing $26,000 of consulting expense over the term of the agreement. On January 4, 2016, the Company issued the 1,000,000 shares of this agreement (See Note 11). The Company has recorded $189 of consulting expense for the six months ended December 31, 2015 related to this agreement.
Shares issued for conversion of convertible debt
On August 14, 2015, pursuant to a conversion notice, $20,500 of principal and interest was converted at $0.02365 into 866,796 shares of common stock (See Note 5).
On August 14, 2015, pursuant to a conversion notice, $20,802 of principal and interest was converted at $0.02365 into 879,585 shares of common stock (See Note 5).
On August 26, 2015, pursuant to a conversion notice, $26,068 of principal and interest was converted at $0.018425 into 1,414,843 shares of common stock (See Note 5).
On September 1, 2015, pursuant to a conversion notice, $25,723 of principal and interest was converted at $0.018425 into 1,396,108 shares of common stock (See Note 5).
On September 4, 2015, pursuant to a conversion notice, $15,648 of principal and interest was converted at $0.018425 into 849,263 shares of common stock (See Note 5).
On September 16, 2015, pursuant to a conversion notice, $15,687 of principal and interest was converted at $0.018975 into 826,726 shares of common stock (See Note 5).
On September 18, 2015, pursuant to a conversion notice, $15,694 of principal and interest was converted at $0.017875 into 877,969 shares of common stock (See Note 5).
On September 22, 2015, pursuant to a conversion notice, $15,638 of principal and interest was converted at $0.01716 into 911,294 shares of common stock (See Note 5).
On October 1, 2015, pursuant to a conversion notice, $26,635 of principal and interest was converted at $0.012375 into 2,152,289 shares of common stock (See Note 5).
On October 7, 2015, pursuant to a conversion notice, $31,374 of principal and interest was converted at $0.012375 into 2,535,293 shares of common stock (See Note 5).
On October 13, 2015, pursuant to a conversion notice, $109,004 of principal and interest was converted at $0.012375 into 8,808,435 shares of common stock (See Note 5).
On October 13, 2015, pursuant to a conversion notice, $104,712 of principal and interest was converted at $0.012375 into 8,461,602 shares of common stock (See Note 5).
100
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
On October 15, 2015, pursuant to a conversion notice, $50,000 of principal and interest was converted at $0.01 into 5,000,000 shares of common stock (See Note 5).
On November 17, 2015, pursuant to a conversion notice, $2,099 of principal and interest was converted at $0.01986 into 105,709 shares of common stock (See Note 5).
On November 17, 2015, pursuant to a conversion notice, $35,000 of principal and interest was converted at $0.01 into 3,500,000 shares of common stock (See Note 5).
On November 23, 2015, pursuant to a conversion notice, $15,707 of principal and interest was converted at $0.0154 into 1,019,925 shares of common stock (See Note 5).
On November 24, 2015, pursuant to a conversion notice, $20,947 of principal and interest was converted at $0.0154 into 1,360,185 shares of common stock (See Note 5).
On November 30, 2015, pursuant to a conversion notice, $49,287 of principal and interest was converted at $0.0154 into 3,200,448 shares of common stock (See Note 5).
On December 4, 2015, pursuant to a conversion notice, $31,703 of principal and interest was converted at $0.0154 into 2,058,637 shares of common stock (See Note 5).
On December 8, 2015, pursuant to a conversion notice, $63,213 of principal and interest was converted at $0.01595 into 3,963,207 shares of common stock (See Note 5).
On December 11, 2015, pursuant to a conversion notice, $50,000 of principal was converted at $0.02608 into 1,917,178 shares of common stock (See Note 5).
On December 15, 2015, pursuant to a conversion notice, $50,000 of principal was converted at $0.02712 into 1,843,658 shares of common stock (See Note 5).
On December 16, 2015, pursuant to a conversion notice, $31,782 of principal and interest was converted at $0.01650 into 1,926,177 shares of common stock (See Note 5).
On December 17, 2015, pursuant to a conversion notice, $40,000 of principal was converted at $0.02500 into 1,600,000 shares of common stock (See Note 5).
On December 21, 2015, pursuant to a conversion notice, $40,000 of principal was converted at $0.02360 into 1,694,916 shares of common stock (See Note 5).
On December 21, 2015, pursuant to a conversion notice, $51,719 of principal and interest was converted at $0.01584 into 3,265,069 shares of common stock (See Note 5).
On December 23, 2015, pursuant to a conversion notice, $40,000 of principal was converted at $0.02320 into 1,724,138 shares of common stock (See Note 5).
On December 23, 2015, pursuant to a conversion notice, $31,414 of principal and interest was converted at $0.01584 into 1,983,188 shares of common stock (See Note 5).
On December 28, 2015, pursuant to a conversion notice, $40,000 of principal was converted at $0.02320 into 1,724,138 shares of common stock (See Note 5).
On December 29, 2015, pursuant to a conversion notice, $15,727 of principal and interest was converted at $0.01573 into 999,783 shares of common stock (See Note 5).
On December 30, 2015, pursuant to a conversion notice, $40,000 of principal was converted at $0.02284 into 1,751,314 shares of common stock (See Note 5).
101
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
Warrants:
On October 28, 2015, pursuant to a convertible debenture, the Company issued 26,190,476 warrants to purchase common stock. These warrants have an exercise price of $0.60 per share and expire 4 years from the date of issuance (See Note 5).
As of December 31, 2015, there were 33,569,634 warrants outstanding and exercisable with expiration dates commencing September 2018 – May 2020.
NOTE 7 – COMMITMENTS AND CONTINGIENCIES
Legal Matters
From time to time, the Company may be involved in litigation relating to claims arising out of the Company’s operations in the normal course of business. The Company is presently in litigation with Typenex Co-Investment, LLC, a Utah limited liability company (“Typenex”), in the Third Judicial District Court of Salt Lake County, Utah. Typenex is claiming funds due under a convertible promissory note dated June 4, 2015. The Company is actively defending all allegations made by Typenex, and has lodged a counter claim against the plaintiff in the Circuit Court of Hillsborough County, Florida. The Company does not believe the result of this litigation matter will have a material adverse effect on our financial conditions or results of operations.
The Company negotiated a settlement with JMJ Financial Inc., a Florida corporation (“JMJ”), and on December 10, 2015, the Company repaid cash of $90,000 as payment in full of the promissory note (See Note 5).
Operating Agreements
In November 2009, the Company entered into a commercialization agreement whereby the Company agreed to pay royalties of 2% of net revenues. Additionally, the Company agreed to pay 5% of each and every license agreement subscribed for. The contract is cancellable at any time by either party. To date, no amounts are owed under the agreement.
Operating Leases
On May 1, 2015, the Company moved to new premises. On May 1, 2015, the Company entered into a month to month lease agreement with new landlord with a monthly rental fee of approximately $2,200 AUD and requiring a three month notice, by either party, to terminate agreement.
Rent expense for the six months ended December 31, 2015 and 2014 were $9,827 and $5,344 respectively.
NOTE 8 – RELATED PARTY TRANSACTIONS
Since inception, Propanc Health Group Corporation has conducted transactions with directors and director related entities. These transactions included the following:
As of December 31, 2015 and June 30, 2015, the Company owed certain directors a total of $54,005 and $79,416 respectively, for money loaned to the Company throughout the years. The loan balance owed at December 31, 2015 was not interest bearing (See Note 4).
As of December 31, 2015 and June 30 2015, the Company owed two directors a total of $33,471 and $35,108, respectively, related to expenses paid on behalf of the Company related to corporate startup costs and intellectual property (See Note 3).
102
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
NOTE 9 – CONCENTRATIONS AND RISKS
Concentration of Credit Risk
The Company maintains its cash in banks and financial institutions in Australia. Bank deposits in Australian banks are uninsured. The Company has not experienced any losses in such accounts through December 31, 2015.
Receivable Concentration
As of December 31, 2015 and June 30, 2015, the Company’s receivables were 100% related to reimbursements on GST taxes paid.
Product and Patent Concentration
As of December 31, 2015 the Company was undertaking preclinical activities for their lead product. The Company was also undertaking research to uncover the mechanism of action of their lead product in order to screen new compounds for development.
The Company previously expanded by the filing of an international PCT patent application (No. PCT/AU2010/001403) directed to enhanced proenzyme formulations and combination therapies. The international PCT application has been based on previous provisional patent applications capturing the Company’s ongoing research and development in this area.
The Company received grant status in South Africa and more recently in Australia and New Zealand. In addition, the United States Patent and Trademark Office or USPTO and European Patent Office or EPO have made preliminary indications that key features of our technology are patentable. The Company is presently working towards securing a patent in each region, covering as many aspects of its technology as possible, while also actively seeking protection throughout Eastern Europe, Asia and South America. Individual countries and regions, include United States, Canada, Japan, Brazil, China, Mexico, Hong Kong, Singapore, Israel, Chile, Peru, Malaysia, Vietnam, Indonesia, Europe, Russia, India, and South Korea. The patent is granted in South Africa, Australia, and New Zealand.
Further provisional patent filings are also expected to be filed to capture and protect additional patentable subject matter that is identified, namely further enhanced formulations, combination treatments, use of recombinant products, modes of action and molecular targets.
Foreign Operations
As of December 31, 2015 and June 30, 2015, the Company's operations are based in Australia.
NOTE 10 - DERIVATIVE FINANCIAL INSTRUMENTS and FAIR VALUE MEASUREMENTS
Derivative Financial Instruments:
The Company applies the provisions of ASC Topic 815-40, Contracts in Entity’s Own Equity (“ASC Topic 815-40”), under which convertible instruments and warrants, which contain terms that protect holders from declines in the stock price (reset provisions), may not be exempt from derivative accounting treatment. As a result, warrants and embedded conversion options in convertible debt are recorded as a liability and are revalued at fair value at each reporting date. If the fair value of the warrants exceeds the face value of the related debt, the excess is recorded as change in fair value in operations on the issuance date. The Company has 3,000,000 warrants and $2,375,024 of convertible debt with repricing options and $60,000 of convertible debt with variable conversion pricing qualifying for derivative accounting treatment outstanding at December 31, 2015.
The Company calculates the estimated fair values of the liabilities for derivative instruments using the Black Scholes (“BSM”) option pricing model. The closing price of the Company’s common stock at December 31, 2015 was $0.0279. Volatility, expected remaining term and risk free interest rates used to estimate the fair value of derivative liabilities at December 31, 2015, are indicated in the table that follows. The volatility was based on historical volatility at December 31, 2015, the expected term is equal to the remaining term of the warrants and the risk free rate is based upon rates for treasury securities with the same term.
103
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
Warrants
| December 31, 2015 | ||||
| Volatility | 296 | % | ||
| Expected remaining term | 2.75 | |||
| Risk-free interest rate | 1.31 | % | ||
| Expected dividend yield | none | |||
Convertible Debt
| Initial Valuations (on new derivative instruments entered into during the three months ended December 31, 2015) | December 31, 2015 | |||||||
| Volatility | 296 | % | 296 | % | ||||
| Expected Remaining Term | 1.00 | 0.26 - 1.19 | ||||||
| Risk Free Interest Rate | 0.7 | % | 0.6 | % | ||||
| Expected dividend yield | none | none | ||||||
Fair Value Measurements:
The Company measures and reports at fair value the liability for derivative instruments. The fair value liabilities for price adjustable warrants and embedded conversion options have been recorded as determined utilizing the BSM option pricing model. The following tables summarize the Company’s financial assets and liabilities measured at fair value on a recurring basis as of December 31, 2015:
| Balance at | Quoted Prices | Significant | ||||||||||||||
| December | in Active | Other | Significant | |||||||||||||
| 31, | Markets for | Observable | Unobservable | |||||||||||||
| 2015 | Identical Assets | Inputs | Inputs | |||||||||||||
| (Level 1) | (Level 2) | (Level 3) | ||||||||||||||
| Embedded conversion option liabilities | $ | 2,511,405 | $ | — | $ | — | $ | 2,511,405 | ||||||||
| Fair value of liability for warrant derivative instruments | $ | 82,086 | $ | — | $ | — | $ | 82,086 | ||||||||
| Total | $ | 2,593,491 | $ | — | $ | — | $ | 2,593,491 | ||||||||
The following is a roll forward for the six months ended December 31, 2015 of the fair value liability of price adjustable derivative instruments:
| Fair Value of | ||||
| Liability for | ||||
| Derivative | ||||
| Instruments | ||||
| Balance at June 30, 2015 | $ | 1,049,929 | ||
| Effects of foreign currency exchange rate changes | (14,253 | ) | ||
| Initial fair value of embedded conversion option derivative liability recorded as debt discount | 1,005,925 | |||
| Initial fair value of embedded conversion option derivative liability recorded as change in fair value of ECO | 1,369,261 | |||
| Change in fair value included in statements of operations | (817,371 | ) | ||
| Balance at December 31, 2015 | $ | 2,593,491 | ||
104
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONDENSED NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015
(unaudited)
NOTE 11 – SUBSEQUENT EVENTS
Shares issued for conversion of convertible debt
On January 4, 2016, pursuant to a conversion notice, $20,995 of principal and interest was converted at $0.0143 into 1,468,187 shares of common stock (See Note 5).
On January 4, 2016, pursuant to a conversion notice, $54,375 of principal was converted at $0.02156 into 2,522,032 shares of common stock (See Note 5).
On January 6, 2016, pursuant to a conversion notice, $40,000 of principal was converted at $0.02068 into 1,934,236 shares of common stock (See Note 5).
On January 6, 2016, pursuant to a conversion notice, $21,004 of principal and interest was converted at $0.014135 into 1,485,946 shares of common stock (See Note 5).
On January 8, 2016, pursuant to a conversion notice, $40,000 of principal was converted at $0.02008 into 1,992,032 shares of common stock (See Note 5).
On January 8, 2016, pursuant to a conversion notice, $10,506 of principal and interest was converted at $0.0113805 into 761,050 shares of common stock (See Note 5).
On January 11, 2016, pursuant to a conversion notice, $10,513 of principal and interest was converted at $0.01375 into 764,573 shares of common stock (See Note 5).
On January 12, 2016, pursuant to a conversion notice, $10,515 of principal and interest was converted at $0.012705 into 827,632 shares of common stock (See Note 5).
On January 13, 2016, pursuant to a conversion notice, $17,650 of principal was converted at $0.01864 into 946,889 shares of common stock (See Note 5).
On January 13, 2016, pursuant to a conversion notice, $10,517 of principal and interest was converted at $0.011825 into 889,409 shares of common stock (See Note 5).
On January 13, 2016, pursuant to a conversion notice, $20,820 of principal and interest was converted at $0.01056 into 1,971,565 shares of common stock (See Note 5).
On January 14, 2016, pursuant to a conversion notice, $82,350 of principal was converted at $0.0168 into 4,901,786 shares of common stock (See Note 5).
On January 19, 2016, pursuant to a conversion notice, $10,423 of principal and interest was converted at $0.009955 into 1,047,013 shares of common stock (See Note 5).
On January 20, 2016, pursuant to a conversion notice, $5,108 of principal and interest was converted at $0.009955 into 513,158 shares of common stock (See Note 5).
On January 21, 2016, pursuant to a conversion notice, $25,000 of principal was converted at $0.01488 into 1,680,108 shares of common stock (See Note 5).
On January 21, 2016, pursuant to a conversion notice, $12,513 of principal and interest was converted at $0.009955 into 1,256,944 shares of common stock (See Note 5).
On January 25, 2016, pursuant to a conversion notice, $25,000 of principal was converted at $0.01492 into 1,675,604 shares of common stock (See Note 5).
On January 25, 2016, pursuant to a conversion notice, $13,567 of principal and interest was converted at $0.009955 into 1,362,834 shares of common stock (See Note 5).
On January 27, 2016, pursuant to a conversion notice, $15,661 of principal and interest was converted at $0.009955 into 1,573,161 shares of common stock (See Note 5).
On January 29, 2016, pursuant to a conversion notice, $25,000 of principal was converted at $0.015080 into 1,657,825 shares of common stock (See Note 5).
On February 1, 2016, pursuant to a conversion notice, $16,722 of principal and interest was converted at $0.009955 into 1,679,800 shares of common stock (See Note 5).
On February 3, 2016, pursuant to a conversion notice, $20,000 of principal was converted at $0.0148 into 1,351,352 shares of common stock (See Note 5).
On February 3, 2016, pursuant to a conversion notice, $10,456 of principal and interest was converted at $0.009405 into 1,111,737 shares of common stock (See Note 5).
On February 4, 2016, pursuant to a conversion notice, $25,000 of principal was converted at $0.0142 into 1,760,564 shares of common stock (See Note 5).
On February 4, 2016, pursuant to a conversion notice, $26,145 of principal and interest was converted at $0.009405 into 2,779,927 shares of common stock (See Note 5).
Shares issued for services
On December 30, 2015, the Company entered into an agreement, effective on January 1, 2016, with a consultant to provide services over a six month period. The Company agreed to issue the consultant 2,250,000 shares of common stock. The Company valued the 2,250,000 shares based on the market price on the effective date of the agreement of $0.0279 and will amortize the $62,775 over the six month term of the agreement. On January 4, 2016, the Company issued 375,000 shares of common stock related to this agreement.
On December 31, 2015, the Company entered into an agreement, effective on January 1, 2016, with a law firm to provide legal services. The Company agreed to issue the law firm 1,600,000 shares of common stock. The Company valued the 1,600,000 shares based on the market price on the effective date of the agreement of $0.0279 and will expense the $44,640. On January 4, 2016, the Company issued 1,600,000 shares of common stock related to this agreement.
On January 4, 2016, the Company issued 1,000,000 shares of common stock to a consultant related to the agreement dated on December 30, 2015 as noted in Note 6.
105
| Page | |
| Fiscal Years Ended June 30, 2015 and 2014 | |
| Report of Independent Registered Public Accounting Firm | F-2 |
| Consolidated Balance Sheets | F-3 |
| Consolidated Statements of Operations and Other Comprehensive Income (Loss) | F-4 |
| Consolidated Statements of Changes in Stockholders’ Deficit | F-5 |
| Consolidated Statements of Cash Flows | F-6 |
| Notes to Consolidated Financial Statements | F-7 |
| F-1 |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of:
Propanc Health Group Corporation
We have audited the accompanying consolidated balance sheets of Propanc Health Group Corporation and Subsidiary at June 30, 2015 and 2014 and the related consolidated statements of operations and other comprehensive income (loss), changes in stockholders’ deficit and cash flows for each of the two years in the period ended June 30, 2015. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Propanc Health Group Corporation and Subsidiary at June 30, 2015 and 2014 and the consolidated results of its operations and its cash flows for each of the two years in the period ended June 30, 2015, in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company had no revenues and had a net loss of $3,412,754 and net cash used in operations of $1,426,479. Additionally, as of June 30, 2015, the company had a working capital deficit, stockholders' deficit and accumulated deficit of $3,058,694, $3,053,516, and $20,965,671, respectively. These matters raise substantial doubt about the Company’s ability to continue as a going concern. Management’s Plan in regards to these matters is also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Salberg & Company, P.A.
SALBERG & COMPANY, P.A.
Boca Raton, Florida
September 30, 2015
2295 NW Corporate Blvd., Suite 240 • Boca Raton, FL 33431-7328
Phone: (561) 995-8270 • Toll Free: (866) CPA-8500 • Fax: (561) 995-1920
www.salbergco.com • info@salbergco.com
Member National Association of Certified Valuation Analysts • Registered with the PCAOB
Member CPAConnect with Affiliated Offices Worldwide • Member AICPA Center for Audit Quality
| F-2 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONSOLIDATED BALANCE SHEETS
| June 30, 2015 | June 30, 2014 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | $ | 107,627 | $ | 87,799 | ||||
| GST tax receivable | 11,647 | 946 | ||||||
| Prepaid expenses and other current assets | 502,616 | 25,000 | ||||||
| TOTAL CURRENT ASSETS | 621,890 | 113,745 | ||||||
| Security deposit | 1,684 | - | ||||||
| Property and equipment, net | 3,494 | - | ||||||
| TOTAL ASSETS | $ | 627,068 | $ | 113,745 | ||||
| LIABILITIES AND STOCKHOLDERS' DEFICIT | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable | $ | 236,466 | $ | 350,004 | ||||
| Accrued expenses and other payables | 386,311 | 422,326 | ||||||
Convertible notes and related accrued interest, net | 1,794,375 | 272,424 | ||||||
| Loans and notes payable | 27,558 | 33,909 | ||||||
| Embedded conversion option liabilities | 780,281 | - | ||||||
| Warrant derivative liability | 269,648 | 158,244 | ||||||
| Due to directors - related parties | 35,108 | 60,350 | ||||||
| Loans from directors and officer - related parties | 79,416 | 161,975 | ||||||
| Employee benefit liability | 71,421 | 62,827 | ||||||
| TOTAL CURRENT LIABILITIES | 3,680,584 | 1,522,059 | ||||||
| Commitments and Contingencies (See Note 9) | ||||||||
| STOCKHOLDERS' DEFICIT: | ||||||||
| Series A preferred stock, $0.01 par value; 10,000,000 shares authorized; 500,000 and 0 shares issued and outstanding as of June 30, 2015 and June 30, 2014, respectively | 5,000 | - | ||||||
| Series B preferred stock, $0.01 par value; 5 shares authorized; 1 and 0 shares issued and outstanding as of June 30, 2015 and June 30, 2014, respectively | - | - | ||||||
| Common stock, $0.001 par value; 2,000,000,000 shares authorized; 347,442,013 and 72,684,767 shares issued and outstanding as of June 30, 2015 and June 30, 2014, respectively | 347,442 | 72,685 | ||||||
| Additional paid-in capital | 17,458,745 | 16,374,781 | ||||||
| Accumulated other comprehensive income (loss) | 100,968 | (302,863 | ) | |||||
| Accumulated deficit | (20,965,671 | ) | (17,552,917 | ) | ||||
| TOTAL STOCKHOLDERS' DEFICIT | (3,053,516 | ) | (1,408,314 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT | $ | 627,068 | $ | 113,745 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-3 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF OPERATIONS AND OTHER COMPREHENSIVE INCOME (LOSS)
| Years Ended June 30, | ||||||||
| 2015 | 2014 | |||||||
| REVENUE | ||||||||
| Revenue | $ | - | $ | - | ||||
| OPERATING EXPENSES | ||||||||
| Administration expenses | 1,567,549 | 742,037 | ||||||
| Occupancy expenses | 3,719 | 11,016 | ||||||
| Research and development | 134,319 | 8,168 | ||||||
| TOTAL OPERATING EXPENSES | 1,705,587 | 761,221 | ||||||
| LOSS FROM OPERATIONS | (1,705,587 | ) | (761,221 | ) | ||||
| OTHER INCOME (EXPENSE) | ||||||||
| Interest expense | (1,323,902 | ) | (93,147 | ) | ||||
| Interest income | 33 | 18 | ||||||
| Other expense | (50,002 | ) | - | |||||
| Change in fair value of derivative liabilities | (541,981 | ) | (16,522 | ) | ||||
| Gain on debt settlements, net | 375,547 | - | ||||||
| Foreign currency transaction loss | (244,332 | ) | (6,959 | ) | ||||
| TOTAL OTHER INCOME (EXPENSE) | (1,784,637 | ) | (116,610 | ) | ||||
| LOSS BEFORE INCOME TAXES | (3,490,224 | ) | (877,831 | ) | ||||
| INCOME TAX BENEFIT | 77,470 | 48,267 | ||||||
| NET LOSS | (3,412,754 | ) | (829,564 | ) | ||||
| OTHER COMPREHENSIVE INCOME (LOSS) | ||||||||
| Foreign currency translation gain (loss) | 403,831 | (58,274 | ) | |||||
| TOTAL OTHER COMPREHENSIVE INCOME (LOSS) | $ | (3,008,923 | ) | $ | (887,838 | ) | ||
| BASIC AND DILUTED NET LOSS PER SHARE | $ | (0.02 | ) | $ | (0.01 | ) | ||
| BASIC AND DILUTED WEIGHTED AVERAGE SHARES OUTSTANDING | 177,633,496 | 72,350,555 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-4 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS' DEFICIT
FOR THE YEARS ENDED JUNE 30, 2015 AND 2014
| Accumulated | ||||||||||||||||||||||||||||||||||||||||||||||||
| Preferred Stock | Other | Total | ||||||||||||||||||||||||||||||||||||||||||||||
| Common Stock Issuable | Series A | Series B | Common Stock | Additional | Comprehensive | Stockholders' | ||||||||||||||||||||||||||||||||||||||||||
| No. of Shares | Value | No. of Shares | Value | No. of Shares | Value | No. of Shares | Value | Paid-in Capital | Accumulated Deficit | Income (Loss) | Deficit | |||||||||||||||||||||||||||||||||||||
| Balance at June 30, 2013 | 25,000 | $ | 25 | - | $ | - | - | $ | - | 70,632,267 | $ | 70,632 | $ | 16,104,809 | $ | (16,723,353 | ) | $ | (244,589 | ) | $ | (792,476 | ) | |||||||||||||||||||||||||
| Issuance of stock for services | (25,000 | ) | (25 | ) | - | - | - | - | 1,915,000 | 1,915 | 242,610 | - | - | 244,500 | ||||||||||||||||||||||||||||||||||
| Issuance of common stock for conversion of accrued expenses | - | - | - | - | - | - | 137,500 | 138 | 27,362 | - | - | 27,500 | ||||||||||||||||||||||||||||||||||||
| Foreign currency translation loss | - | - | - | - | - | - | - | - | - | - | (58,274 | ) | (58,274 | ) | ||||||||||||||||||||||||||||||||||
| Net loss, 2014 | - | - | - | - | - | - | - | - | - | (829,564 | ) | - | (829,564 | ) | ||||||||||||||||||||||||||||||||||
| Balance at June 30, 2014 | - | $ | - | - | $ | - | - | $ | - | 72,684,767 | $ | 72,685 | $ | 16,374,781 | $ | (17,552,917 | ) | $ | (302,863 | ) | $ | (1,408,314 | ) | |||||||||||||||||||||||||
| Issuance of common stock for cash | - | - | - | - | - | - | 13,400,000 | 13,400 | 15,600 | - | - | 29,000 | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock for conversion of convertible debt | - | - | - | - | - | - | 181,185,110 | 181,185 | 259,975 | - | - | 441,160 | ||||||||||||||||||||||||||||||||||||
| Issuance of stock for services | - | - | 500,000 | 5,000 | 1 | - | 19,258,316 | 19,258 | 717,018 | - | - | 741,276 | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock as part of settlement agreement | - | - | - | - | - | - | 60,913,820 | 60,914 | 91,371 | - | - | 152,285 | ||||||||||||||||||||||||||||||||||||
| Foreign currency translation gain | - | - | - | - | - | - | - | - | - | - | 403,831 | 403,831 | ||||||||||||||||||||||||||||||||||||
| Net loss, 2015 | - | - | - | - | - | - | - | - | - | (3,412,754 | ) | - | (3,412,754 | ) | ||||||||||||||||||||||||||||||||||
| Balance at June 30, 2015 | - | $ | - | 500,000 | $ | 5,000 | 1 | $ | - | 347,442,013 | $ | 347,442 | $ | 17,458,745 | $ | (20,965,671 | ) | $ | 100,968 | $ | (3,053,516 | ) | ||||||||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Years Ended June 30, | ||||||||
| 2015 | 2014 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (3,412,754 | ) | $ | (829,564 | ) | ||
| Adjustments to Reconcile Net Loss to Net Cash Used in Operating Activities: | ||||||||
| Issuance of common stock for services and voluntary ratchet | 276,792 | 237,833 | ||||||
| Issuance of preferred stock for services | 1,067 | - | ||||||
| Fair value of warrants issued for services | 51,488 | - | ||||||
Gain on debt settlements, net | (375,547 | ) | - | |||||
| Settlement fees paid in the form of debt | 150,000 | - | ||||||
| Amortization of prepaid shares issued for services | - | 6,667 | ||||||
| Foreign currency transaction loss | 31,548 | - | ||||||
| Depreciation expense | 81 | 538 | ||||||
| Amortization of debt discount | 295,795 | 49,029 | ||||||
| Change in fair value of derivative liabilities | 541,981 | 16,522 | ||||||
| Accretion of put premium | 1,044,196 | 27,187 | ||||||
| Changes in Assets and Liabilities: | ||||||||
| GST receivable | (10,879 | ) | - | |||||
| Prepaid expenses and other assets | (74,303 | ) | 294 | |||||
| Accounts payable | (47,977 | ) | 94,607 | |||||
| Employee benefit liability | 20,362 | 11,598 | ||||||
| Accrued expenses | 115,941 | 158,847 | ||||||
| Accrued interest | (34,270 | ) | - | |||||
| NET CASH USED IN OPERATING ACTIVITIES | (1,426,479 | ) | (226,442 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Payment for security deposit | (1,684 | ) | - | |||||
| Purchase of equipment | (3,901 | ) | - | |||||
| NET CASH USED IN INVESTING ACTIVITIES | (5,585 | ) | - | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Bank overdraft | - | (6 | ) | |||||
| Loan repayments to principal stockholder | (28,455 | ) | (45,512 | ) | ||||
| Proceeds from convertible promissory notes | 1,438,500 | 273,959 | ||||||
| Repayments of convertible promissory notes | (157,000 | ) | - | |||||
| Proceeds from issuance of common stock for cash | 29,000 | - | ||||||
| Loan proceeds | - | 10,542 | ||||||
| Loan proceeds from principal stockholder | - | 72,158 | ||||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 1,282,045 | 311,141 | ||||||
| Effect of exchange rate changes on cash | 169,847 | 3,100 | ||||||
| NET INCREASE IN CASH | 19,828 | 87,799 | ||||||
| CASH AT BEGINNING OF YEAR | 87,799 | - | ||||||
| CASH AT END OF YEAR | $ | 107,627 | $ | 87,799 | ||||
| Supplemental Disclosure of Cash Flow Information | ||||||||
| Cash paid during the year: | ||||||||
| Interest | $ | - | $ | - | ||||
| Income Tax | $ | - | $ | - | ||||
| Supplemental Disclosure of Non-Cash Investing and Financing Activities | ||||||||
| Common stock issued for settlement of debt | $ | 152,285 | $ | - | ||||
| Prepaid common stock issued for services | $ | 269,682 | $ | 6,667 | ||||
| Prepaid warrants issued for services | $ | 138,314 | $ | - | ||||
| Reduction of put premium related to conversions of convertible note | $ | 71,370 | $ | - | ||||
| Conversion of accrued expenses to common stock | $ | - | $ | 27,500 | ||||
| Conversion of convertible notes and accrued interest to common stock | $ | 374,771 | $ | - | ||||
| Discounts related to warrants issued with convertible debenture | $ | - | $ | 133,095 | ||||
| Discounts related to lender costs | $ | 48,500 | $ | 30,000 | ||||
| Discounts related to derivative liability | $ | 305,000 | $ | - | ||||
| Conversion of loan payable to convertible debenture | $ | - | $ | 27,963 | ||||
| Conversion of loan payable to common stock | $ | 66,389 | $ | - | ||||
| Prepaid settlement fee paid through issuance of convertible note | $ | - | $ | 25,000 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-6 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
NOTE 1 – NATURE OF OPERATIONS AND SUMMARY OF SIGNIFICANT ACCOUNTING AND REPORTING POLICIES
Nature of Operations
Propanc PTY LTD was incorporated in Melbourne, Victoria Australia on October 15, 2007, and is based in Richmond, Victoria Australia. Since inception, substantially all of the efforts of the Company have been the development of new cancer treatments targeting high risk patients who need a follow up, nontoxic, long term therapy which prevents the cancer from returning and spreading. The Company anticipates establishing global markets for its technologies.
On November 23, 2010, Propanc Health Group Corporation ("the Company", "we", "us", "our") was incorporated in the state of Delaware. In January 2011, to reorganize the Company, Propanc Health Group Corporation acquired all of the outstanding shares of Propanc PTY LTD on a one-for-one basis making it a wholly-owned subsidiary.
Principals of Consolidation
The consolidated financial statements include the accounts of Propanc Health Group Corporation and its wholly-owned subsidiary, Propanc PTY LTD. All significant inter-company balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates. Significant estimates in the accompanying consolidated financial statements include the estimates of useful lives for depreciation, valuation of derivatives, valuation of beneficial conversion features on convertible debt, allowance for uncollectable receivables, valuation of equity based instruments issued for other than cash, the valuation allowance on deferred tax assets and foreign currency translation due to certain average exchange rates applied in lieu of spot rates on transaction dates.
Foreign Currency Translation and Comprehensive Income (Loss)
The Company’s functional currency is the Australian dollar (AUD). For financial reporting purposes, the Australian dollar has been translated into United States dollars ($) and/or USD as the reporting currency. Assets and liabilities are translated at the exchange rate in effect at the balance sheet date. Revenues and expenses are translated at the average rate of exchange prevailing during the reporting period. Equity transactions are translated at each historical transaction date spot rate. Translation adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’ equity (deficit) as “accumulated other comprehensive income (loss).” Gains and losses resulting from foreign currency transactions are included in the statement of operations and comprehensive loss as other income (expense). There have been no significant fluctuations in the exchange rate for the conversion of Australian dollars to USD after the balance sheet date.
Comprehensive income (loss) for all periods presented, includes only foreign currency translation gains (losses).
Changes in Accumulated Other Comprehensive Income (Loss) by component during the years ended June 30, 2015 and 2014 were as follows:
| Foreign Currency Items: | ||||
| Beginning balance, June 30, 2013 | $ | (244,589 | ) | |
| Foreign currency translation loss | (58,274 | ) | ||
| Balance, June 30, 2014 | (302,863 | ) | ||
| Foreign currency translation gain | 403,831 | |||
| Ending balance, June 30, 2015 | $ | 100,968 | ||
Fair Value of Financial Instruments and Fair Value Measurements
The Company measures their financial assets and liabilities in accordance with US GAAP. For certain of the Company’s financial instruments, including cash and cash equivalents, accounts and other receivables, accounts payable and accrued expenses and other liabilities, the carrying amounts approximate fair value due to their short maturities. Amounts recorded for loans payable, also approximate fair value because current interest rates available to us for debt with similar terms and maturities are substantially the same.
| F-7 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
The Company adopted accounting guidance for fair value measurements of financial assets and liabilities. The adoption did not have a material impact on the Company’s results of operations, financial position or liquidity. This standard defines fair value, provides guidance for measuring fair value and requires certain disclosures. This standard does not require any new fair value measurements, but rather applies to all other accounting pronouncements that require or permit fair value measurements. This guidance does not apply to measurements related to share-based payments. This guidance discusses valuation techniques, such as the market approach (comparable market prices), the income approach (present value of future income or cash flow), and the cost approach (cost to replace the service capacity of an asset or replacement cost). The guidance utilizes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The following is a brief description of those three levels:
Level 1: Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2: Inputs other than quoted prices that are observable, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active.
Level 3: Unobservable inputs in which little or no market data exists, therefore developed using estimates and assumptions developed by us, which reflect those that a market participant would use.
Cash and Cash Equivalents
Cash and cash equivalents include cash on hand and at banks, short-term deposits with an original maturity of three months or less with financial institutions, and bank overdrafts. Bank overdrafts are reflected as a current liability on the balance sheets. There were no cash equivalents as of June 30, 2015 or 2014.
Receivables
As amounts become uncollectible, they will be charged to an allowance and operations in the period when a determination of uncollectability is made. Any estimates of potentially uncollectible customer accounts receivable will be made based on an analysis of individual customer and historical write-off experience. The Company’s analysis included the age of the receivable account, creditworthiness of the customer and general economic conditions.
Property, Plant, and Equipment
Property and equipment are stated at cost, net of accumulated depreciation. Expenditures for maintenance and repairs are expensed as incurred; additions, renewals, and betterments are capitalized. When property and equipment are retired or otherwise disposed of, the related cost and accumulated depreciation are removed from the respective accounts, and any gain or loss is included in operations. Depreciation of property and equipment is provided using the declining balance method. The depreciable amount is the cost less its residual value.
The estimated useful lives are as follows:
Machinery and equipment 5 years
Patents
Patent costs are stated at cost and reclassified to intangible assets and amortized on a straight-line basis over the estimated future periods if and once the patent has been granted by a regulatory agency, however, the Company will expense any costs as long as the Company is in the startup stage. Accordingly, as the Company's product was and is not currently approved for market, thus any patent costs incurred from 2013 through 2015 were expensed immediately. Currently, the Company has one International patent pending which was jointly applied for by the Company and another entity.
The Company received grant status, or been accepted in South Africa, Australia, and New Zealand. In addition, the United States Patent and Trademark Office or USPTO and European Patent Office or EPO have made preliminary indications that key features of the Company’s technology are patentable. The Company is presently working towards securing a patent in each region, covering as many aspects of its technology as possible, whilst also actively seeking protection throughout Eastern Europe, Asia and South America.
Individual countries and regions where the Company is actively seeking patent protection include United States, Canada, Japan, Brazil, China, Mexico, Hong Kong, Singapore, Israel, Chile, Peru, Malaysia, Vietnam, Indonesia, Europe, Russia, India, and South Korea. The patent is now granted, or accepted in South Africa, Australia, and New Zealand.
| F-8 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
Impairment of Long-Lived Assets
In accordance with ASC 360-10, Long-lived assets, which include property and equipment and intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of long-lived assets to be held and used is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the assets. Fair value is generally determined using the asset’s expected future discounted cash flows or market value, if readily determinable.
Employee Benefit/Liability
Liabilities arising in respect of wages and salaries, annual leave, accumulated sick leave and any other employee benefits expected to be settled within twelve months of the reporting date are measured at their nominal amounts based on remuneration rates which are expected to be paid when the liability is settled. All other employee benefit liabilities are measured at the present value of the estimated future cash outflow to be made in respect of services provided by employees up to the reporting date. All employee liabilities are owed within the next twelve months and therefore, recorded at nominal value.
Australian Goods and Services Tax (GST)
Revenues, expenses and balance sheet items are recognized net of the amount of GST except payable and receivable balances which are shown inclusive of GST. The GST incurred is payable on revenues to, and recoverable on purchases from, the Australian Taxation Office.
Cash flows are presented in the statements of cash flow on a gross basis, except for the GST component of investing and financing activities, which are disclosed as operating cash flows.
As of June 30, 2015 and June 30, 2014 the Company was owed $11,647 and $946 from the Australian Taxation Office. These amounts were fully collected subsequent to the balance sheet reporting dates.
Derivative Instruments
ASC Topic 815, Derivatives and Hedging (“ASC Topic 815”), establishes accounting and reporting standards for derivative instruments and for hedging activities by requiring that all derivatives be recognized in the balance sheet and measured at fair value. Gains or losses resulting from changes in the fair value of derivatives are recognized in earnings or recorded in other comprehensive income (loss) depending on the purpose of the derivatives and whether they qualify and have been designated for hedge accounting treatment. The Company does not have any derivative instruments for which it has applied hedge accounting treatment.
Convertible Notes With Variable Conversion Options
The Company has entered into convertible notes, some of which contain variable conversion options, whereby the outstanding principal and accrued interest may be converted, by the holder, into common shares at a fixed discount to the price of the common stock at the time of conversion. The Company treats these convertible notes as stock settled debt under ASC 480 and measures the fair value of the notes at the time of issuance, which is the result of the share price discount at the time of conversion, and records the put premium as accretion to interest expense to the date of first conversion.
Income Taxes
The Company is governed by Australia and United States income tax laws, which are administered by the Australian Taxation Office and the United States Internal Revenue Service, respectively. The Company follows FASB ASC 740 when accounting for income taxes, which requires an asset and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed annually for temporary differences between the financial statements and tax bases of assets and liabilities that will result in taxable or deductible amounts in the future based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Income tax expense is the tax payable or refundable for the period plus or minus the change during the period in deferred tax assets and liabilities.
The Company adopted provisions of ASC 740, Sections 25 through 60, “Accounting for Uncertainty in Income Taxes." These sections provide detailed guidance for the financial statement recognition, measurement and disclosure of uncertain tax positions recognized in the financial statements. Tax positions must meet a “more-likely-than-not” recognition threshold at the effective date to be recognized upon the adoption of ASC 740 and in subsequent periods. Upon the adoption of ASC 740, the Company had no unrecognized tax benefits. During the years ended June 30, 2015 and 2014 no adjustments were recognized for uncertain tax benefits. The years 2008 through 2015 are subject to examination by the Australian Taxation Office. The years ended June 30, 2012 through 2015 is subject to examination by the United States Internal Revenue Service.
| F-9 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
Research and Development Costs and Tax Credits
In accordance with ASC 730-10, research and development costs are expensed when incurred. Total research and development costs for the years ended June 30, 2015 and 2014 were $134,319 and $8,168 respectively.
The Company may apply for research and development tax concessions with the Australian Taxation Office on an annual basis. Although the amount is possible to estimate at year end, the Australian Taxation Office may reject or materially alter the claim amount. Accordingly, the Company does not recognize the benefit of the claim amount until cash receipt since collectability is not certain until such time. The tax concession is a refundable credit. If the Company has net income then the Company can receive the credit which reduces its income tax liability. If the Company has net losses then the Company may still receive a cash payment for the credit, however, the Company's net operating loss carryforwards are reduced by the gross equivalent loss that would produce the credit amount when the income tax rate is applied to that gross amount. The concession is recognized as an income tax benefit, in operations, upon receipt.
During the years ended June 30, 2015 and 2014, the Company applied for and received from the Australian Taxation Office a research and development tax credit in the amount of $77,470 and $48,267 respectively, which is reflected as an income tax benefit in the accompanying consolidated statements of operations and other comprehensive income (loss).
Stock Based Compensation
The Company records stock based compensation in accordance with ASC section 718, “Stock Compensation” and Staff Accounting Bulletin (SAB) No. 107 (SAB 107) issued by the SEC in March 2005 regarding its interpretation of ASC 718. ASC 718 requires the fair value of all stock-based employee compensation awarded to employees to be recorded as an expense over the related requisite service period. The Company values any employee or non-employee stock based compensation at fair value using the Black-Scholes Option Pricing Model.
The Company accounts for non-employee share-based awards in accordance with the measurement and recognition criteria of ASC 505-50 “Equity-Based Payments to Non-Employees”.
Revenue Recognition
In accordance with SEC Staff Accounting Bulletin (SAB) No. 104, Revenue Recognition, (codified in ASC 605) the Company recognizes revenue when (i) persuasive evidence of a customer or distributor arrangement exists or acceptance occurs, (ii) a retailer, distributor or wholesaler receives the goods, (iii) the price is fixed or determinable, and (iv) collectability of the sales revenues is reasonably assured. Subject to these criteria, the Company recognizes revenue relating to royalties on product sales in the period in which the sale occurs and the royalty term has begun.
Start-up Costs
In accordance with ASC 720-15-15, start-up costs are expensed as incurred.
Basic and Diluted Net Loss Per Common Share
Basic net loss per share is computed by dividing the net loss by the weighted average number of common shares outstanding during the period. Diluted net loss per common share is computed by dividing the net loss by the weighted average number of common shares outstanding for the period and, if dilutive, potential common shares outstanding during the period. Potentially dilutive securities consist of the incremental common shares issuable upon exercise of common stock equivalents such as stock options, warrants and convertible debt instruments. Potentially dilutive securities are excluded from the computation if their effect is anti-dilutive. As a result, the basic and diluted per share amounts for all periods presented are identical. For the years ended June 30 2015 and 2014, there were 7,379,158 and 3,000,000 warrants respectively outstanding and fourteen and four convertible notes payable that are convertible into 335,716,597 and 6,069,667 common shares respectively which are considered dilutive securities which were excluded from the computation since the effect is anti-dilutive.
Recently Adopted Accounting Pronouncements
Financial Accounting Standards Board, Accounting Standard Updates which are not effective until after June 30, 2015 are not expected to have a significant effect on the Company’s consolidated financial position or results of operations. The Company implemented the following at June 30, 2015:
In June 2014, the FASB issued ASU No. 2014-10, which amended Accounting Standards Codification (ASC) Topic 915 Development Stage Entities. The amendment eliminates certain financial reporting requirements surrounding development stage entities, including an amendment to the variable interest entities guidance in ASC Topic 810, Consolidation. The amendment removes the definition of a development stage entity from the ASC, thereby removing the financial reporting distinction between development stage entities and other entities from U.S. GAAP. Consequently, the amendment eliminates the requirements for development stage entities to (1) present inception-to-date information in the statements of income, cash flows and shareholder equity, (2) label the financial statements as those of a development stage entity, (3) disclose a description of the development stage activities in which the entity is engaged, and (4) disclose the first year in which the entity is no longer a development stage entity that in prior years it had been in the development stage.
| F-10 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
This amendment is effective for fiscal years beginning after December 15, 2014, and interim periods therein. Early application of each of the amendments is permitted for any annual reporting period or interim period for which the entity's financial statements have not yet been issued. The Company has made the election to early adopt this amendment effective June 30, 2014 and, as a result, the Company is no longer presenting or disclosing the information previously required under Topic 915. The early adoption was made to reduce data maintenance by removing all incremental financial reporting requirements for development stage entities. The adoption of this amendment alters the disclosure requirements of the Company, but it does not have any material impact on the Company's financial position or results of operations for the current or any prior reporting periods.
In August 2014, the FASB issued ASU 2014-15, “Presentation of Financial Statements – Going Concern (Topic 205-40)”, which requires management to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern for each annual and interim reporting period. If substantial doubt exists, additional disclosure is required. This new standard will be effective for the Company for annual and interim periods beginning after December 15, 2016. Early adoption is permitted. The Company expects to adopt this new standard as of December 31, 2015. The Company does not expect this ASU to have a material impact on its consolidated financial statements.
On May 8, 2015, the FASB issued ASU 2015-08, “Business Combinations (Topic 805) Pushdown Accounting” which conforms the FASB’s guidance on pushdown accounting with the SEC’s guidance. ASU 2015-08 is effective for annual periods beginning after December 15, 2015. The Company does not expect this ASU to have a material impact on its consolidated financial statements.
In April 2015, the Financial Accounting Standards Board issued Accounting Standards Update No. 2015-03, "Simplifying the Presentation of Debt Issuance Costs," which changes the presentation of debt issuance costs in financial statements. Under this guidance such costs would be presented as a direct deduction from the related debt liability rather than as an asset. This guidance is effective for interim and annual reporting periods beginning after December 15, 2015. The Company is currently evaluating the impact this guidance will have on its Consolidated Balance Sheet, but expects that as of June 30, 2015 this guidance would not have a material effect on the consolidated balances current presentation.
NOTE 2 – GOING CONCERN
The accompanying consolidated financial statements have been prepared in conformity with US GAAP, which contemplate continuation of the Company as a going concern. For the year ended June 30, 2015, the Company had no revenues and had a net loss of $3,412,754 and net cash used in operations of $1,426,479. Additionally, as of June 30, 2015, the Company had a working capital deficit, stockholders' deficit and accumulated deficit of $3,058,694, $3,053,516, and $20,965,671 respectively. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements do not include any adjustments to reflect the possible future effect on the recoverability and classification of assets or the amounts and classifications of liabilities that may result from the outcome of this uncertainty.
Successful completion of the Company’s development program and, ultimately, the attainment of profitable operations are dependent upon future events, including obtaining adequate financing to fulfill its development activities, acceptance of the Company's International patent application and achieving a level of sales adequate to support the Company’s cost structure. However, there can be no assurances that the Company will be able to secure additional equity investment or achieve an adequate sales level.
NOTE 3 – PROPERTY AND EQUIPMENT
Property, plant, and equipment consist of the following as of June 30,
| 2015 | 2014 | |||||||
| Office equipment at cost | $ | 15,732 | $ | 14,968 | ||||
| Less: Accumulated depreciation | (12,238 | ) | (14,968 | ) | ||||
| Total property, plant, and equipment | $ | 3,494 | $ | - | ||||
Depreciation expense for the years ended June 30, 2015 and 2014 were $81 and $538 respectively.
| F-11 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
NOTE 4 – DUE TO DIRECTORS - RELATED PARTIES
Due to directors - related parties represents unsecured advances made by the directors for operating expenses on behalf of the Company such as intellectual property and formation expenses. The expenses were paid for on behalf of the Company and are due upon demand. The Company is currently not being charged interest under these advances. The total amount owed these directors at June 30, 2015 and June 30, 2014 is $35,108 and $60,350 respectively. As part of the settlement and stipulation agreement noted in Note 9, the Company reduced this liability by approximately $44,000. On January 30, 2015, as part of the Settlement and Lock-up Agreement, the above agreement was terminated and the Company increased this liability by approximately $44,000. On February 4, 2015, the Company entered into a Debt Settlement Agreement with some of these directors whereby the Company issued shares of common stock as settlement of approximately $14,000 of the balance due to these directors (See Note 5).
NOTE 5 – LOANS AND NOTES PAYABLE
Loans from Directors and Officer - Related Parties
Loans from Directors and an Officer at June 30, 2015 and June 30, 2014 were $79,416 and $161,975, respectively. The loans beared no interest and were all past their due date and in default. As part of the settlement and stipulation agreement noted in Note 9, the Company reduced this liability by approximately $127,000. On January 30, 2015, as part of the Settlement and Lock-up Agreement, the above agreement was terminated and the Company increased this liability by approximately $109,000. On February 4, 2015, the Company entered into a Debt Settlement Agreement with some of these directors whereby the Company issued 33,259,350 and 17,654,470 shares of common stock as settlement of approximately $27,000 of the balance due to these directors and $14,000 of debt as discussed in Note 4. The Company valued the common stock at a price of $0.0025 per share based on the last private placement purchase price per share for a total value of $127,284 which resulted in the Company recording a loss of $86,455 as a result of these settlements. During the year ended June 30, 2015, the Company made loan repayments of approximately $28,500.
Other Loans from Unrelated Parties
Loans from two unrelated parties were received during 2013 totaling $33,614. They bear interest at 10% and as of June 30, 2014 one was past its due date and in default and the other, with a September 30, 2013 balance of $27,963 was exchanged for a convertible debenture as discussed below in Note 6.
A loan from an unrelated party was received during the year ended June 30, 2014 totaling $9,419. It bears interest at 10%.
A loan from an unrelated party was received during the year ended June 30, 2014 totaling $18,839 and is non-interest bearing.
Accordingly, other loans totaled $33,909 at June 30, 2014.
In July 2014, the loans and notes payable balance of $33,909 as of June 30, 2014 was removed as part of the settlement and stipulation agreement and consolidated into one loan (See Note 9). On January 30, 2015, as part of the Settlement and Lock-up Agreement, the above agreement was terminated and the Company reclassified the remaining other loans from unrelated parties balance. As of June 30, 2015, the other loans from unrelated parties balance was $27,558.
Debt Settlement to be Paid in Stock, Net of Premium
In July 2014, the Company consolidated outstanding debt and other liabilities as part of a settlement agreement (See Note 9) and was indebted to one unrelated party for approximately $1,033,000 which includes a $50,000 note payable issued as a fee to the lender, a $355,000 premium and $628,000 of principal. On September 11, 2014 and on November 4, 2014, the Company issued 7,426,000 and 8,161,000 shares of common stock as a settlement of a portion of that debt for a total value of $81,396 (See Note 8). On January 30, 2015, as part of the Settlement and Lock-up Agreement with the lender, the above agreement was terminated and the Company reclassified remaining principal outstanding debts and other liabilities of approximately $575,000 back to the original debt holders. In addition, since this agreement was terminated the Company wrote off the remaining premium of approximately $310,000 to gain on debt settlement and the $50,000 note payable issued as a fee and $17,000 premium as a gain on debt settlement.
Notes Payable
On July 18, 2014, the Company paid a $50,000 fee to the investor (See Note 9) in the form of a $50,000 promissory note, non-interest bearing and due January 31, 2015. On January 30, 2015, the Company entered into a Settlement and Lock-up Agreement with a lender whereby the Company issued 10,000,000 shares of common stock as settlement of the $50,000 promissory note issued on July 18, 2014 in connection with an Equity Purchase Agreement of the same date and a $25,000 convertible promissory note issued in connection with a Settlement and Stipulation Agreement dated May 2014 and accrued interest of $1,466. The Company valued the common stock at a price of $0.0025 per share based on the last private placement purchase price per share for a total value of $25,000 which resulted in the Company recording a gain of $51,466 as a result of this settlement.
| F-12 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
NOTE 6 – CONVERTIBLE NOTES
Convertible notes at June 30, 2015 and 2014 were as follows:
| June 30, 2015 | June 30, 2014 | |||||||
| Convertible notes and debenture | $ | 1,455,000 | $ | 366,296 | ||||
| Unamortized discounts | (415,467 | ) | (121,059 | ) | ||||
| Accrued interest | 26,989 | - | ||||||
| Premium, net | 727,853 | 27,187 | ||||||
| Convertible notes, net | $ | 1,794,375 | $ | 272,424 | ||||
On September 30, 2013 the Company’s subsidiary issued a Debenture for $139,680 (AUD $150,000) plus warrants for 3,000,000 common shares of the Company. The Company agreed to pay 12% interest on the principal amount and the maturity date is December 31, 2015. This debenture rolls into it $27,963 of loans outstanding at June 30, 2013, an August 2013 note of $63,196 along with September advances of $46,446 and accrued interest. The debenture is convertible only at the Company’s option into common stock at $0.075 AUD per share and is convertible at that same rate by the lender only upon default by the Company, as defined in the debenture. The warrants were determined to be derivative instruments due to the variable exercise price of the warrants which is initially $0.0698 and subject to adjustment if the Company issues shares at a price below the initial exercise price. Accordingly, the fair value of the warrants was determined using a Black-Scholes option pricing model with a stock price of $0.20, exercise price of $0.075 AUD, volatility of 53% based on the comparative companies method since the Company’s stock is very thinly traded, an expected term of 27 months based on the debenture term and a risk free rate of 0.4%. The approximate initial $400,000 value of the warrants was recorded as a derivative liability in the accompanying consolidated balance sheet, along with a debt discount of approximately $140,000 and change in warrant derivative liability of approximately $260,000 as an expense for the three months ended September 30, 2013. (See Note 12 for current period re-measurement) On July 2, 2014, this $139,680 convertible debenture and accrued interest of $15,118 was converted, using the contractual conversion rate of $0.0709 or $0.075 AUD, into 2,183,333 shares of the Company’s common stock (See Note 8).
On May 8, 2014, the Company issued a 10% convertible promissory note for $25,000 as a prepaid fee for services to be provided under a settlement and stipulation agreement as discussed in Note 9. The note and all accrued interest was due on November 8, 2014 and was in default. The note is convertible immediately at 50% of the lowest closing bid price in the 30 trading days prior to conversion. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $25,000 put premium which was fully expensed during the year ended June 30, 2015. On January 30, 2015, this note principal of $25,000 and accrued interest of $1,466 was settled as part of a Settlement and Lock-Up Agreement (See Note 9).
On May 29, 2014, the Company issued a convertible note payable for $75,000. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is May 29, 2015. The note is convertible at the option of the holder at any time after 180 days at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $61,364 put premium over 180 days from the execution of the convertible note. During the year ended June 30, 2015, the Company has accreted the remaining $51,089 of the put premium as $10,275 had been accreted at June 30, 2014, resulting in the put premium being fully expensed. During the year ended June 30, 2015, the Company converted $14,547 of principal and accrued interest of $218 into shares of the Company’s common stock (See Note 8). Additionally, $61,364 of the put premium was expensed as interest expense and the remaining $60,453 of principal and $4,352 of accrued interest was assigned to a third party. As of June 30, 2015, this note was fully converted.
On May 29, 2014, the Company issued a second convertible note payable for $75,000. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is May 29, 2015. The note is convertible at the option of the holder at any time after 180 days at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $61,364 put premium over 180 days from the execution of the convertible note. During the year ended June 30, 2015, the Company has accreted the remaining $51,089 of the put premium as $10,275 had been accreted at June 30, 2014, resulting in the put premium being fully expensed. During the year ended June 30, 2015, the Company converted $11,755 of principal and accrued interest of $553 into shares of the Company’s common stock (See Note 8). Additionally, $61,364 of the put premium was expensed as interest expense and the remaining $63,245 of principal and $3,313 of accrued interest was assigned to a third party. As of June 30, 2015, this note was fully converted.
On May 30, 2014, the Company issued a third convertible note payable for $50,000. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is May 30, 2015. The note is convertible at the option of the holder at any time after 180 days at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $40,909 put premium over 180 days from the execution of the convertible note. During the year ended June 30, 2015, the Company has accreted the remaining $34,273 of the put premium as $6,636 had been accreted at June 30, 2014, resulting in the put premium being fully expensed. During the year ended June 30, 2015, the Company converted $50,000 of principal and accrued interest of $3,346 into shares of the Company’s common stock (See Note 8). Additionally, this conversion resulted in a $40,909 reduction of the put premium. As of June 30, 2015, this note was fully converted.
| F-13 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
In addition to each of the above initial convertible promissory notes (“initial convertible notes”), the Company issued to each lender another convertible promissory note for the same amounts of $75,000, $75,000 and $50,000 termed "Back-End Notes". These notes have the same terms as the initial convertible notes. Each Back-End Note shall initially be paid for by an offsetting promissory note issued to the Company by the lender ("Note Receivable") provided that prior to the conversion of the Back-End Notes, the holders must have paid off the Notes Receivable in cash. The Notes Receivable were due on January 30, 2015, unless the Company does not meet the “current public information” requirement pursuant to Rule 144, in which case both the Back-End Notes and the Notes Receivable may both be cancelled. The Notes Receivable are initially secured by the pledge of the Back-End Notes, but may be exchanged for other collateral with an appraised value of at least $50,000, upon Company’s approval following a three (3) day written notice to the Company. The term of the Notes Receivable and the Back-End Notes are one year, upon which the outstanding principal and interest is payable. The amounts funded plus accrued interest under Back-End Notes are convertible into common stock at any time after the requisite Rule 144 holding period (subject to the condition above for the Back-End Notes), at a conversion price equal to 55% of the lowest trading bid price in the ten (10) trading days prior to the conversion. The $50,000 Back-End Note was issued as noted below.
In the event the Company redeems the initial convertible notes in full, the Company is required to pay off all principal, interest and any other amounts owing multiplied by i) 130% if prepaid within 60 days of the issuance date; ii) 140% if prepaid 60 but less than 121 days after the issuance date; and (iii) 150% if prepaid 120 but less than 180 days after the issuance date. There shall be no redemption after the 180th day. The Back-End Notes may not be prepaid, except that if the initial convertible notes are redeemed by the Company within six months of their issuance, all obligations of the Company and holders under the Back-End Notes and the Notes Receivable will be deemed satisfied and such notes shall automatically be deemed cancelled and of no further force or effect.
In the event of two specific defaults, which include the maintenance of a minimum trading price and an aggregate dollar trading volume of the Company's common shares, the holders may cancel the Back-End Notes and the related Notes Receivable and otherwise in the event of other defaults as defined in the securities purchase agreement, the amount of principal and accrued interest will become immediately due and payable and may be offset by amounts due to the Company by the holders. Additionally, the Back-End Notes will bear default interest at a rate of 16% per annum, or the highest rate of interest permitted by law.
Since the Back-End Notes are not convertible until the Notes Receivable are paid and also not for 180 days from the note dates, and the Notes Receivable and Back-End Notes have a right of setoff, the Notes Receivable and Back-End Notes and related accrued interest receivable and payable have been netted for presentation purposes on the accompanying consolidated balance sheet.
On August 6, 2014 (execution date), the Company executed a convertible promissory note in the principal sum of $250,000, with an original issue discount (“OID”) of $25,000. The consideration to be paid to the Lender shall be equal to the consideration actually paid by the Lender plus prorated interest and any other fees that the Company shall be required to pay. The original issue discount shall also be prorated based on the actual consideration received to equal approximately 10% of the consideration received. If the Company repays a consideration payment on or before the first 90 days from the effective date of that payment, the interest rate on that payment of consideration will be 0%. If the Company does not repay a payment on or before the 90 days, the Company will incur a one-time interest charge of 12% on the principal amount of the loan. Upon execution of the note, the note holder made an initial payment of $25,000 (net of a $2,500 OID) to the Company of the total consideration. The maturity date is two years from the date of each payment to the Company, and is the date upon which the principal sum, as well as any unpaid interest and other fees, shall be due and payable. The note is convertible, at the option of the investor, to common stock of the Company at any time after the effective date at the lesser of $0.09 or 60% of the lowest trade price in the 25 trading days prior to the conversion. This note was bifurcated with the embedded conversion option recorded as a derivative liability at fair value (See Note 12). As of June 30, 2015, the Company didn’t repay the consideration and therefore incurred a 12% interest charge. Accrued interest as of June 30, 2015 was $2,134 and no further funding has been received under the $250,000 note.
On November 17, 2014, the Company issued a convertible promissory note for $43,000. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is August 20, 2015. The note is convertible at the option of the holder at any time after 180 days at a rate of 58% of the average lowest three trading closing bid prices of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $31,138 put premium over 180 days from the execution of the convertible note. During the year ended June 30, 2015, the Company has accreted $27,851 of the put premium. During the year ended June 30, 2015, the Company repaid cash of $61,632 as payment in full of $43,000 of principal and accrued interest of $1,527 resulting in $17,105 of a prepayment penalty which was expensed as interest expense. Additionally, this repayment resulted in a $3,287 reduction of the remaining put premium. As of June 30, 2015, this note was paid in full.
On December 10, 2014, the Company issued a convertible promissory note for $28,000. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is September 12, 2015. The note is convertible at the option of the holder at any time after 180 days at a rate of 58% of the average lowest three trading closing bid prices of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $20,276 put premium over 180 days from the execution of the convertible note. During the year ended June 30, 2015, the Company has accreted $15,657 of the put premium. During the year ended June 30, 2015, the Company repaid cash of $38,654 as payment in full of $28,000 of principal and accrued interest of $853 resulting in $9,801 of a prepayment penalty which was expensed as interest expense. Additionally, this repayment resulted in a $4,619 reduction of the remaining put premium. As of June 30, 2015, this note was paid in full.
| F-14 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
On January 26, 2015, the Company issued a convertible promissory note for $28,000. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is October 28, 2015. The note is convertible at the option of the holder at any time after 180 days at a rate of 58% of the average lowest three trading closing bid prices of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $20,276 put premium over 180 days from the execution of the convertible note. During the year ended June 30, 2015, the Company has accreted $15,432 of the put premium. During the year ended June 30, 2015, the Company repaid cash of $37,137 as payment in full of $28,000 of principal and accrued interest of $835 resulting in $8,302 of a prepayment penalty which was expensed as interest expense. Additionally, this repayment resulted in a $4,844 reduction of the remaining put premium. As of June 30, 2015, this note was paid in full.
On January 27, 2015, the Company received payment of the Note Receivable of $50,000 that offsets the Back-End Note that was issued on May 30, 2014. Proceeds from the Note Receivable of $7,779, $2,500 and $5,000 were paid directly to the stock transfer agent, legal fees and capital raising fees respectively resulting in net cash proceeds of $34,721 received by the Company. This Back-End Note is related to the initial convertible note that was issued on May 30, 2014 and has the same terms as previously discussed. As a result, the Back-End Note is now eligible for conversion at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $40,909 put premium over 180 days from the execution of the convertible note. During the year ended June 30, 2015, the Company has accreted $40,909 of the put premium resulting in the put premium being fully expensed. During the year ended June 30, 2015, the Company converted $50,000 of principal and accrued interest of $609 into shares of the Company’s common stock (See Note 8). Additionally, this conversion resulted in a $40,909 reduction of the put premium. As of June 30, 2015, this note was fully converted.
On February 10, 2015, the Company issued a convertible note payable for $45,000 with an OID of $7,500. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is February 10, 2016. The note is convertible at the option of the holder at any time after 180 days at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days prior to the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $36,818 put premium over 180 days from the execution of the convertible note. Through June 30, 2015, the Company has accreted $27,409 of the put premium. Accrued interest as of June 30, 2015 was $1,391.
On February 17, 2015, the Company issued a second convertible note payable for $45,000 with an OID of $7,500. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is February 17, 2016. The note is convertible at the option of the holder at any time after 180 days at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days prior to the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $36,818 put premium over 180 days from the execution of the convertible note. Through June 30, 2015, the Company has accreted $27,409 of the put premium. Accrued interest as of June 30, 2015 was $1,322.
On March 12, 2015, the Company issued a third convertible note payable for $170,500 with an OID of $13,000. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is March 12, 2016. The note is convertible at the option of the holder at any time at a rate of 55% of the Company’s common stock for the average of the lowest three trading prices in the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company recognized a $139,500 put premium. Accrued interest as of June 30, 2015 was $4,148.
On March 20, 2015, the Company issued a fourth convertible note payable for $150,000. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is March 20, 2016. The note is convertible at the option of the holder at any time at a rate of 55% of the lowest trading bid price of the Company’s common stock for the average of the lowest three trading priced in the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company recognized a $122,727 put premium. Accrued interest as of June 30, 2015 was $3,386.
In addition to each of the above initial convertible promissory notes (“initial convertible notes”), the Company issued to each lender another convertible promissory note for the same amounts of $45,000, $45,000, $170,500 and $150,000 termed "Back-End Notes". These notes have the same terms as the initial convertible notes. Each Back-End Note shall initially be paid for by an offsetting promissory note issued to the Company by the lender ("Note Receivable") provided that prior to the conversion of the Back-End Notes, the holders must have paid off the Notes Receivable in cash. Each Note Receivable is due eight months from issuance of each initial convertible note, unless the Company does not meet the “current public information” requirement pursuant to Rule 144, in which case both the Back-End Notes and the Notes Receivable may both be cancelled. Each Note Receivable is initially secured by the pledge of the Back-End Notes, but may be exchanged for other collateral with an appraised value of at least the principal amount of the note less the OID, upon Company’s approval following a three (3) day written notice to the Company. The term of the Notes Receivable and the Back-End Notes are one year, upon which the outstanding principal and interest is payable. The amounts funded plus accrued interest under Back-End Notes are convertible into common stock at any time after the requisite Rule 144 holding period (subject to the condition above for the Back-End Notes), at a conversion price equal to 55% of the lowest trading bid price in the ten (10) trading days prior to the conversion. The $45,000, $45,000, $170,500 and $150,000 Back-End Notes were issued as noted below.
| F-15 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
In the event the Company redeems the initial convertible notes in full, the Company is required to pay off all principal, interest and any other amounts owing multiplied by i) 130% if prepaid within 60 days of the issuance date; ii) 140% if prepaid 60 but less than 121 days after the issuance date; and (iii) 150% if prepaid 120 but less than 180 days after the issuance date. There shall be no redemption after the 180th day. The Back-End Notes may not be prepaid, except that if the initial convertible notes are redeemed by the Company within six months of their issuance, all obligations of the Company and holders under the Back-End Notes and the Notes Receivable will be deemed satisfied and such notes shall automatically be deemed cancelled and of no further force or effect.
In the event of two specific defaults, which include the maintenance of a minimum trading price and an aggregate dollar trading volume of the Company's common shares, the holders may cancel the Back-End Notes and the related Notes Receivable and otherwise in the event of other defaults as defined in the securities purchase agreement, the amount of principal and accrued interest will become immediately due and payable and may be offset by amounts due to the Company by the holders. Additionally, the Back-End Notes will bear default interest at a rate of 24% per annum, or the highest rate of interest permitted by law.
Since the Back-End Notes are not convertible until the Notes Receivable are paid and also not for 180 days from the note dates, and the Notes Receivable and Back-End Notes have a right of setoff, the Notes Receivable and Back-End Notes and related accrued interest receivable and payable have been netted for presentation purposes on the accompanying consolidated balance sheet.
On February 15, 2015, in connection with a six-month consulting agreement, the Company issued a convertible promissory note for $90,000 as compensation for services to be rendered. The Company agreed to pay 5% interest per annum on the principal amount and the maturity date is August 15, 2015. The note is convertible at the option of the holder at any time after issuance of note at a rate of 60% of the lowest trading price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company fully expensed a $60,000 put premium. Accrued interest as of June 30, 2015 was $1,677.
On February 20, 2015, the Company issued a convertible promissory note for $58,000. The Company agreed to pay 12% interest per annum on the principal amount and the maturity date is July 27, 2015. The note is convertible at the option of the holder at any time after 180 days at a rate of 50% of the average lowest three trading closing bid prices of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $58,000 put premium over 180 days from the execution of the convertible note. During the year ended June 30, 2015, the Company has accreted $36,411 of the put premium. During the year ended June 30, 2015, the Company repaid cash of $83,512 as payment in full of $58,000 of principal and accrued interest of $2,212 resulting in $23,300 of a prepayment penalty which was expensed as interest expense. Additionally, this repayment resulted in a $21,589 reduction of the remaining put premium. As of June 30, 2015, this note was paid in full.
On March 12, 2015, the Company issued a convertible promissory note for $104,000. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is December 16, 2015. The note is convertible at the option of the holder at any time after 180 days at a rate of 58% of the average lowest three trading closing bid prices of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $75,310 put premium over 180 days from the execution of the convertible note. Through June 30, 2015, the Company has accreted $46,441 of the put premium. Accrued interest as of June 30, 2015 was $2,530. On July 15, 2015, the Company repaid cash of $137,915 as payment in full of $104,000 of principal and accrued interest of $2,872 (See Note 13).
On March 12, 2015, in connection with a two-year consulting agreement, the Company issued a convertible promissory note for $60,000 as compensation for services to be rendered. The Company agreed to pay 10% interest per annum on the principal amount and the maturity date is March 11, 2017. The note is convertible, at the option of the holder, at any time after the effective date at the lesser of $0.0175 or 75% of the volume weighted average of the lowest three trading closing bid prices of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. This note was bifurcated with the embedded conversion option recorded as a derivative liability at fair value (See Note 12). Accrued interest as of June 30, 2015 was $1,825.
On April 20, 2015, the Company issued a convertible note payable for $17,500. The Company agreed to pay 8% interest per annum on the principal amount and the maturity date is April 20, 2016. The note is convertible at the option of the holder at any time at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company recognized a $14,318 put premium. Accrued interest as of June 30, 2015 was $272.
On April 24, 2015, the Company received payment of the Note Receivable of $45,000, less the OID of $7,500, that offsets the Back-End Note that was issued on February 10, 2015. Proceeds from the Note Receivable of $2,250 were paid directly to legal fees resulting in net cash proceeds of $35,250 received by the Company. This Back-End Note is related to the initial convertible note that was issued on February 10, 2015 and has the same terms as previously discussed. As a result, the Back-End Note is now eligible for conversion at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $36,818 put premium over 180 days from the execution of the convertible note. Through June 30, 2015, the Company has accreted $13,909 of the put premium. Accrued interest as of June 30, 2015 was $671.
| F-16 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
On April 24, 2015, the Company received payment of the Note Receivable of $45,000, less the OID of $7,500, that offsets the Back-End Note that was issued on February 17, 2015. Proceeds from the Note Receivable of $2,250 were paid directly to legal fees resulting in net cash proceeds of $35,250 received by the Company. This Back-End Note is related to the initial convertible note that was issued on February 17, 2015 and has the same terms as previously discussed. As a result, the Back-End Note is now eligible for conversion at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company is accreting a $36,818 put premium over 180 days from the execution of the convertible note. Through June 30, 2015, the Company has accreted $13,909 of the put premium. Accrued interest as of June 30, 2015 was $671.
On April 27, 2015, the Company received payment of the Note Receivable of $170,500, less the OID of $13,000, that offsets the Back-End Note that was issued on March 12, 2015. Proceeds from the Note Receivable of $7,500 were paid directly to legal fees resulting in net cash proceeds of $150,000 received by the Company. This Back-End Note is related to the initial convertible note that was issued on March 12, 2015 and has the same terms as previously discussed. As a result, the Back-End Note is now eligible for conversion at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company recognized a $139,500 put premium. Accrued interest as of June 30, 2015 was $2,429.
On May 19, 2015, the Company entered into a Securities Purchase Agreement (“SPA”), to issue a series of nine back end convertible notes in the principal sum of $782,500, pursuant to the SPA, the Company issued to the lender nine convertible promissory notes termed "Back-End Notes", in the amounts of $37,500 ("Back-End Note 1"), $37,500 ("Back-End Note 1")$37,500 ("Back-End Note 1"), $37,500 ("Back-End Note 2"), $157,500 ("Back-End Note 3"), $150,000 ("Back-End Note 4"), $17,500 ("Back-End Note 5"), $37,500 ("Back-End Note 6"), $37,500 ("Back-End Note 7"), $157,500 ("Back-End Note 8") and $150,000 ("Back-End Note 9"). These notes have the same terms as the initial convertible notes. Each Back-End Note shall initially be paid for by an offsetting promissory note issued to the Company by the lender ("Note Receivable") provided that prior to the conversion of the Back-End Notes, the holders must have paid off the Notes Receivable in cash. Each Note Receivable is due on May 19, 2016, unless the Company does not meet the “current public information” requirement pursuant to Rule 144, in which case both the Back-End Notes and the Notes Receivable may both be cancelled. Each Note Receivable is initially secured by the pledge of the Back-End Notes, but may be exchanged for other collateral with an appraised value of at least the principal amount of the note less the OID, upon Company’s approval following a three (3) day written notice to the Company. The term of the Notes Receivable and the Back-End Notes are one year, upon which the outstanding principal and interest is payable. The amounts funded plus accrued interest under Back-End Notes are convertible into common stock at any time after the requisite Rule 144 holding period (subject to the condition above for the Back-End Notes), at a conversion price equal to 55% of the lowest trading bid price in the ten (10) trading days prior to the conversion. See Note 13 as no funds were received under these notes until after June 30, 2015.
The Back-End Notes may not be prepaid, except that if the initial convertible notes are redeemed by the Company within six months of their issuance, all obligations of the Company and holders under the Back-End Notes and the Notes Receivable will be deemed satisfied and such notes shall automatically be deemed cancelled and of no further force or effect.
In the event of two specific defaults, which include the maintenance of a minimum trading price and an aggregate dollar trading volume of the Company's common shares, the holders may cancel the Back-End Notes and the related Notes Receivable and otherwise in the event of other defaults as defined in the securities purchase agreement, the amount of principal and accrued interest will become immediately due and payable and may be offset by amounts due to the Company by the holders. Additionally, the Back-End Notes will bear default interest at a rate of 24% per annum, or the highest rate of interest permitted by law.
Since the Back-End Notes are not convertible until the Notes Receivable are paid, and the Notes Receivable and Back-End Notes have a right of setoff, the Notes Receivable and Back-End Notes and related accrued interest receivable and payable have been netted for presentation purposes on the accompanying consolidated balance sheet.
On June 2, 2015, the Company received payment of the Note Receivable of $150,000 that offsets the Back-End Note that was issued on March 20, 2015. Proceeds from the Note Receivable of $7,500 were paid directly to legal fees resulting in net cash proceeds of $142,500 received by the Company. This Back-End Note is related to the initial convertible note that was issued on March 20, 2015 and has the same terms as previously discussed. As a result, the Back-End Note is now eligible for conversion at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received. The convertible note is treated as stock settled debt under ASC 480 and accordingly the Company recognized a $122,727 put premium. Accrued interest as of June 30, 2015 was $2,137.
| F-17 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
On June 4, 2015 (execution date), the Company executed a convertible promissory note in the principal sum of $1,215,000, with an OID of $110,000. The consideration to be paid to the lender shall be equal to the consideration actually paid by the lender plus prorated interest and any other fees that the Company shall be required to pay. The original issue discount shall also be prorated based on the actual consideration received to equal approximately 10% of the consideration received. The Company agreed to pay 10% interest per annum on the principal amount and the maturity date is ten months from the date of each payment to the Company, and is the date upon which the principal sum, as well as any unpaid interest and other fees, shall be due and payable. The note is comprised of an initial cash purchase of $335,000 (includes $30,000 of OID and $5,000 for legal fees) (“Initial Note”), a Secured Investor Note of $220,000 (includes $20,000 of OID) (“Secured Investor Note”) and three Investor Notes of $220,000 each (include $20,000 of OID each) (“Investor Notes”). The Secured Investor Note is secured by the lender’s 40% membership interest in a certain LLC. The Company will accrue 10% interest per annum on the unpaid principal amount of the Secured Investor Note and the three Investor Notes as defined in the agreements. Upon execution of the note, the note holder made an initial cash payment of $300,000 (net of a $30,000 OID and $5,000 for legal fees) to the Company of the total consideration and issued the Secured Investor Note and three Investor Notes to the Company. The Initial Note is convertible, at the option of the lender, to common stock of the Company at any time after the effective date at a price of $0.07 per share, which represents fair value at execution date. This note was determined to be a derivative instrument due to the variable conversion price of the note which is initially $0.07 and subject to adjustment if the Company’s market capitalization falls below $3,000,000 at any time. This note was bifurcated with the embedded conversion option recorded as a derivative liability at fair value (See Note 12). Accrued interest as of June 30, 2015 was $2,513. Since the Secured Investor Note and Investor Notes are not convertible until they are paid in cash to the Company and also not for 180 days from the note dates, the remaining principal of this note and the Secured Investor Note and Investor Notes and related accrued interest receivable and payable have been netted for presentation purposes on the accompanying consolidated balance sheet.
The Company recorded $529,500 and $30,000 of debt discounts for fees paid to lenders related to the above note issuances in 2015 and 2014 respectively. The debt discounts are being amortized over the term of the debt. Amortization of the debt discounts for the years ended June 30, 2015 and 2014 was $114,033 and $3,133 respectively.
NOTE 7 – INCOME TAXES
The Company follows ASC 740-10-10, under which an entity recognizes deferred tax assets and liabilities for future tax consequences or for events that were previously recognized in the Company’s financial statements or tax returns. The measurement of deferred tax assets and liabilities is based on enacted tax law provisions. The effects of future changes in tax laws or rates are not anticipated. As of June 30, 2015, the Company operated exclusively in Australia. The Company was wholly subject to Australia income tax laws and regulations, which are administered by the Australian Taxation Office for the years ended June 30, 2015 and 2014 and all prior years.
On November 23, 2010, Propanc Health Group Corporation was incorporated in the state of Delaware. In January 2011, Propanc Health Group Corporation acquired all of the outstanding shares of Propanc PTY LTD on a one-for-one basis making it a wholly-owned subsidiary. As a result of these transactions, the Company is subject to the income tax laws of both the United States and Australia for the years ended June 30, 2015 and 2014. For the years ended June 30, 2015 and 2014, all the Company’s loss before income taxes resulted entirely from its Australian activities and its taxable loss was only subject to Australian tax law.
At June 30, 2015, the Company has a net operating loss (NOL) for Australian tax purposes only, that approximates $12,486,000. Consequently, the Company may have NOL carryforwards available for income tax purposes, which will continue to be available until they are recovered through earning taxable income. Deferred tax assets would arise from the recognition of anticipated utilization of these net operating losses to offset future taxable income. The NOL is subject to a reduction of $1,527,228 for research and development credits granted by the Australian Taxation Office through June 30, 2015.
The components for the provision for income taxes are as follows:
| Year Ended | ||||||||
| June 30, | June 30, | |||||||
| 2015 | 2014 | |||||||
| Current Taxes | $ | (77,470 | ) | $ | (48,267 | ) | ||
| Deferred Taxes | - | - | ||||||
| Income Taxes Expense (Benefit) | $ | (77,470 | ) | $ | (48,267 | ) | ||
The items accounting for the difference between income taxes at the Australia statutory rate and the provision for income taxes are as follows:
| Year Ended | ||||||||||||||||
| June 30, | June 30, | |||||||||||||||
| 2015 | 2014 | |||||||||||||||
| Amount | Impact on Rate |
Amount | Impact on Rate |
|||||||||||||
| Income Tax Expense (Benefit) at Australia Statutory Rate | $ | (672,087 | ) | (19.26 | )% | $ | (274,229 | ) | (31.24 | )% | ||||||
| Expenses Paid by Parent on Behalf of Foreign Subsidiary | 156,410 | 4.48 | % | 92,480 | 10.54 | % | ||||||||||
| R&D Refundable Tax Credit | (77,470 | ) | (2.22 | )% | (48,267 | ) | (5.50 | )% | ||||||||
| Reduction of NOL Carryforward Due to R&D Tax Credit | 77,470 | 2.22 | % | 48,267 | 5.50 | % | ||||||||||
| Change in Deferred Tax Valuation Allowance | (355,636 | ) | (10.19 | )% | 260,533 | 29.68 | % | |||||||||
| Foreign Exchange Rate Changes | 793,843 | 22.74 | % | (127,051 | ) | (14.47 | )% | |||||||||
| Total Income Tax Expense (Benefit) | $ | (77,470 | ) | (2.22 | )% | $ | (48,267 | ) | (5.50 | )% | ||||||
| F-18 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and amounts used for income tax purposes. Significant components of the Company's net deferred income taxes are as follows:
| June 30, | June 30, | |||||||
| 2015 | 2014 | |||||||
| Current Deferred Tax Assets | ||||||||
| Warrant Derivative Liability | $ | 88,204 | $ | 28,209 | ||||
| Provision for Annual Leave | 21,426 | 18,848 | ||||||
| Superannuation | - | 3,815 | ||||||
| Total Current Deferred Tax Assets | $ | 109,630 | $ | 50,872 | ||||
| Current Deferred Tax Liabilities | ||||||||
| Prepaid Investor Services | $ | - | $ | - | ||||
| Prepaid Expenses | - | - | ||||||
| Prepaid Insurance | - | - | ||||||
| Accounts Payable/Trade Creditors | - | - | ||||||
| Patent Costs | - | - | ||||||
| Total Current Deferred Tax Liabilities | $ | - | $ | - | ||||
| Non-Current Deferred Tax Assets | ||||||||
| Prepaid Investor Services | $ | 185,025 | $ | 751,917 | ||||
| Capital Raising Costs | 23,261 | 28,622 | ||||||
| Legal Costs | 23,583 | 29,019 | ||||||
| Intellectual Property | 11,612 | 14,288 | ||||||
| Patent Costs | 59,995 | 59,473 | ||||||
| Formation Expense | 7,117 | 8,757 | ||||||
| Net Operating Loss Carryover | 3,426,149 | 3,259,060 | ||||||
| Foreign Exchange Loss (OCI) | (30,290 | ) | 90,859 | |||||
| Total Non-Current Deferred Tax Assets | 3,706,452 | 4,241,995 | ||||||
| Deferred Tax Valuation Allowance | (3,816,082 | ) | (4,292,867 | ) | ||||
| Total Non-Current Deferred Tax Assets | (109,630 | ) | (50,872 | ) | ||||
| Total Deferred Tax Assets (Net) | $ | - | $ | - | ||||
Management has determined that the realization of the net deferred tax asset is not assured and has created a valuation allowance for the entire amount of such benefits.
The Company follows ASC 740-10, which provides guidance for the recognition and measurement of certain tax positions in an enterprise’s financial statements. Recognition involves a determination whether it is more likely than not that a tax position will be sustained upon examination with the presumption that the tax position will be examined by the appropriate taxing authority having full knowledge of all relevant information.
The Company’s policy is to record interest and penalties associated with unrecognized tax benefits as additional income taxes in the statement of operations. As of June 30, 2015 the Company had no unrecognized tax benefits. There were no changes in the Company’s unrecognized tax benefits during the years ended June 30, 2015 and 2014. The Company did not recognize any interest or penalties during fiscal 2015 or 2014 related to unrecognized tax benefits.
The income tax returns filed for the tax years from inception will be subject to examination by the relevant taxing authorities.
NOTE 8 – STOCKHOLDERS’ DEFICIT
Preferred Stock:
The total number of preferred shares authorized and that may be issued by the Company is 10,000,000 preferred shares with a par value of $0.01. These preferred shares have no rights to dividends, profit sharing or liquidation preferences.
| F-19 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
Of the total preferred shares authorized, pursuant to Certificate of Designation filed on December 9, 2014, 500,000 have been designated as Series A preferred stock, with a par value of $0.01 (“Series A Preferred Stock”). On December 9, 2014, the Company issued 500,000 shares of Series A Preferred Stock to its CEO in consideration for services rendered to the Company, including for and as an incentive to continue to assist and provide services to the Company. The shares were valued at $0.00213 per share for a total value of $1,067 based on the average sale price per share of the 8,161,000 shares of common stock sold during the three months ended December 31, 2014.
Of the total preferred shares authorized, pursuant to Certificate of Designation filed on June 16, 2015, up to five (5) shares have been designated as Series B preferred stock, with a par value of $0.01 (“Series B Preferred Stock”). Each holder of outstanding shares of Series B Preferred Stock shall be entitled to voting power equivalent of the number of votes equal to the total number of Company’ common stock outstanding as of the record date for the determination of stockholders entitled to vote at each meeting of stockholders of the Company and entitled to vote on all matters submitted or required to be submitted to a vote of the stockholders of the Company. On June 16, 2015, the Company issued 1 share of Series B Preferred Stock to its CEO in consideration for services rendered to the Company, including for and as an incentive to continue to assist and provide services to the Company. The share was valued at $0.1165 per share for a total value of $0.12 based on the closing price of the stock on that date. This value represents the economic rights of the share as the value of voting rights, which represent control rights, are not objectively measureable.
Common Stock:
On November 12, 2014, the Company filed an amendment to the Company’s Certificate of Incorporation with the Secretary of State of the State of Delaware, to increase the Company’s authorized common stock from one hundred million (100,000,000) shares of common stock, par value $0.001 per share, to ten billion (10,000,000,000) shares of common stock, par value $0.001 per share. On July 10, 2015, the Company filed an amendment to the Company’s Certificate of Incorporation with the Secretary of State of the State of Delaware, to decrease the Company’s authorized common stock from ten billion (10,000,000,000) shares of common stock, par value $0.001 per share, to two billion (2,000,000,000) shares of common stock, par value $0.001 per share.
In July 2013, the Company issued 300,000 shares of common stock to a consultant related to a June 6, 2013 agreement. The shares were valued at $0.20 per share (based on current market price) and accordingly, the Company recognized an expense of $12,000 during the first quarter of fiscal 2014 and $48,000 was previously recognized during fiscal 2013 as the expense was amortized over the term of the agreement.
In July 2013, the Company issued 250,000 shares of common stock to a consultant for past services. The shares are fully vested and valued at $0.20 per share (based on current market price) and accordingly, the Company recognized an expense of $50,000 related to the share issuance.
In July 2013, the Company issued 137,500 shares of common stock to a consultant in exchange for a $27,500 accounts payable relating to past services. The shares are fully vested and valued at $0.20 per share (based on current market price) and accordingly there was no gain or loss on this settlement.
In July 2013, the Company issued 10,000 shares of common stock to a consultant for past services. The shares are fully vested and valued at $0.20 per share (based on current market price) and accordingly, the Company recognized an expense of $2,000 related to the share issuance.
In July 2013, the Company issued 150,000 shares of common stock to a consultant for past services. The shares are fully vested and valued at $0.20 per share (based on current market price) and accordingly, the Company recognized an expense of $30,000 related to the share issuance.
In September 2013, the Company issued the balance of 300,000 shares of common stock to a consultant related to a June 6, 2013 agreement. The shares were valued at $0.20 per share (based on current market price) and accordingly, the Company recognized an expense of $60,000 during the three months ended September 30, 2013.
On September 30, 2013, pursuant to a consulting agreement, the company issued 25,000 shares of common stock for past services performed during the quarter. The shares were valued at $0.20 per share (based on current market price) and accordingly, the Company recognized an expense of $5,000 during the three months ended September 30, 2013.
In October 2013, the Company issued 500,000 vested shares of common stock as a non-refundable retainer in conjunction with a 90-day investment banking services agreement. The shares were valued at the market price on the day of the grant, $0.10, and the Company recorded an expense of $50,000.
In October 2013, the Company issued 200,000 shares of common stock to a consultant for services. The shares were issued to the consultant and vest over the three year term of the agreement. The shares were valued at $0.10 per share (based on current market price) and accordingly, the Company recognized an expense of $13,333 and recorded $6,667 in prepaid expenses for January's services which has since been fully expensed.
| F-20 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
In October 2013, the Company issued 100,000 shares of common stock to a consultant for past services. The shares are fully vested and valued at $0.10 per share (based on current market price) and accordingly, the Company recognized an expense of $10,000 related to the share issuance.
In November 2013, the Company issued 30,000 shares of common stock to a consultant for past services. The shares are fully vested and valued at $0.10 per share (based on current market price) and accordingly, the Company recognized an expense of $3,000 related to the share issuance.
In November 2013, the Company issued 25,000 shares of common stock to a consultant for past services. The shares are fully vested and valued at $0.10 per share (based on current market price) and accordingly, the Company recognized an expense of $2,500 related to the share issuance.
On May 9, 2014, the Company entered into an agreement with a consultant to provide services over a twelve month period in exchange for 1,000,000 shares of common stock. The Company valued the 1,000,000 shares based on the market price on the agreement date of $0.10 and will recognize $100,000 of consulting expense through the term of the agreement. On August 7, 2014 the Company issued the first 500,000 shares of this agreement. On April 28, 2015 the Company issued the remaining 500,000 shares of this agreement. The Company has recorded $100,000 of consulting expense as of June 30, 2015 related to this agreement.
On July 2, 2014, a $139,680 convertible note was converted into shares of common stock pursuant to a conversion notice. $154,798 of principal and interest was converted at $0.0709 into 2,183,333 shares (See Note 6).
On September 11, 2014, the Company issued 7,426,000 shares of common stock as the first tranche of a settlement agreement. (See Note 9).
On October 17, 2014, the Company entered into an agreement with a consultant to provide services over a six month period. The Company agreed to issue the consultant 4,000,000, 3,000,000 and 3,000,000 shares of common stock in the first, third and fifth months respectively. The Company valued the 10,000,000 shares based on the market price on the agreement date of $0.008 and is recognizing $80,000 of consulting expense through the term of the agreement. On December 4, 2014, the Company issued the first 4,000,000 shares of this agreement. On April 15, 2015 the Company issued the remaining 6,000,000 shares of this agreement. The Company has recorded $80,000 of consulting expense as of June 30, 2015 related to this agreement.
On November 4, 2014, the Company issued 8,161,000 shares of common stock as the second tranche of a settlement agreement. (See Note 9).
On November 5, 2014, the Company entered into a private placement securities purchase agreement with an accredited investor pursuant to which the Company agreed to issue up to 3,000,000 shares of its common stock at a price of $0.001 per share for an aggregate purchase price of $3,000 in gross proceeds. On December 4, 2014, the Company issued 3,000,000 shares of common stock. There are no registration rights with regards to these securities.
On December 9, 2014, pursuant to a conversion notice, $5,357 of principal and interest was converted at $0.0011 into 4,870,391 shares of common stock (See Note 6).
On December 10, 2014, pursuant to a conversion notice, $7,368 of principal and interest was converted at $0.0011 into 6,698,331 shares of common stock (See Note 6).
On December 11, 2014, the Company entered into a private placement securities purchase agreement with an accredited investor pursuant to which the Company agreed to issue up to 1,000,000 shares of its common stock at a price of $0.0025 per share for an aggregate purchase price of $2,500 in gross proceeds.
On December 16, 2014, the Company entered into a private placement securities purchase agreements with accredited investors pursuant to which the Company agreed to issue up to 9,400,000 shares of its common stock at a price of $0.0025 per share for an aggregate purchase price of $23,500 in gross proceeds.
On December 16, 2014, pursuant to a conversion notice, $6,000 of principal was converted at $0.0012 into 5,194,805 shares of common stock (See Note 6).
On December 24, 2014, pursuant to a conversion notice, $3,762 of principal was converted at $0.0007 into 5,700,000 shares of common stock (See Note 6).
On December 26, 2014, pursuant to a conversion notice, $4,044 of principal and interest was converted at $0.0007 into 5,655,958 shares of common stock (See Note 6).
During the year ended June 30, 2015, pursuant to the January 30, 2015 Settlement and Lock-up Agreement (Note 9), the Company issued 10,000,000 shares of common stock at a rate of $0.0025 per share or $25,000.
| F-21 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
On February 2, 2015, pursuant to a conversion notice, $3,446 of principal and interest was converted at $0.0006 into 6,265,964 shares of common stock (See Note 6).
On February 4, 2015, pursuant to debt settlement agreements with two directors (Note 4), the Company issued 17,654,470 and 33,259,350 shares of common stock valued at $0.0025 per share or $44,136 and $83,148, respectively.
On February 9, 2015, pursuant to a conversion notice, $21,100 of principal and interest was converted at $0.0035 into 6,089,544 shares of common stock (See Note 6).
On February 17, 2015, pursuant to a conversion notice, $3,266 of principal and interest was converted at $0.0006 into 5,937,563 shares of common stock (See Note 6).
On February 17, 2015, pursuant to a conversion notice, $15,323 of principal and interest was converted at $0.0035 into 4,422,257 shares of common stock (See Note 6).
On March 6, 2015, pursuant to a conversion notice, $3,410 of principal was converted at $0.0006 into 6,200,000 shares of common stock (See Note 6).
On March 6, 2015, pursuant to a conversion notice, $6,443 of principal and interest was converted at $0.0015 into shares 4,338,384 of common stock (See Note 6).
On March 6, 2015, pursuant to a conversion notice, $1,011 of principal and interest was converted at $0.0015 into 680,485 shares of common stock (See Note 6).
On March 11, 2015, pursuant to a conversion notice, $14,675 of principal and interest was converted at $0.0015 into 9,882,013 shares of common stock (See Note 6).
On March 11, 2015, pursuant to a conversion notice, $10,121 of principal and interest was converted at $0.0015 into 6,815,185 shares of common stock (See Note 6).
On March 12, 2015, pursuant to a conversion notice, $1,001 of principal and interest was converted at $0.0015 into 674,141 shares of common stock (See Note 6).
On March 12, 2015, pursuant to a conversion notice, $14,678 of principal and interest was converted at $0.0015 into 9,884,155 shares of common stock (See Note 6).
On March 12, 2015, pursuant to a conversion notice, $10,125 of principal and interest was converted at $0.0016 into 6,347,918 shares of common stock (See Note 6).
On March 16, 2015, pursuant to a conversion notice, $15,534 of principal and interest was converted at $0.0017 into 9,110,833 shares of common stock (See Note 6).
On March 17, 2015, pursuant to a conversion notice, $1,061 of principal and interest was converted at $0.0017 into 622,504 shares of common stock (See Note 6).
On March 17, 2015, pursuant to a conversion notice, $20,048 of principal and interest was converted at $0.0019 into 10,414,660 shares of common stock (See Note 6).
On March 18, 2015, pursuant to a conversion notice, $20,053 of principal and interest was converted at $0.0020 into 10,127,576 shares of common stock (See Note 6).
On March 19, 2015, pursuant to a conversion notice, $8,260 of principal and interest was converted at $0.0020 into 4,171,808 shares of common stock (See Note 6).
On March 20, 2015, pursuant to a conversion notice, $23,762 of principal and interest was converted at $0.0020 into 12,001,242 shares of common stock (See Note 6).
On April 14, 2015, pursuant to a conversion notice, $4,271 of principal and interest was converted at $0.0020 into 2,135,450 shares of common stock (See Note 6).
| F-22 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
On April 15, 2015, pursuant to a conversion notice, $10,145 of principal and interest was converted at $0.0020 into 5,072,740 shares of common stock (See Note 6).
On April 21, 2015, pursuant to a conversion notice, $28,202 of principal and interest was converted at $0.0020 into 14,100,870 shares of common stock (See Note 6).
On May 7, 2015, the Company entered into an agreement with a consultant to provide services over a six month period in exchange for 6,758,316 shares of common stock. The Company valued the 6,758,316 shares based on the market price on the agreement date of $0.043 and will recognize $290,608 of consulting expense through the term of the agreement. On June 5, 2015 the Company issued the 6,758,316 shares of this agreement. The Company has recorded $88,446 of consulting expense as of June 30, 2015 related to this agreement.
On May 21, 2015, the Company entered into an agreement with a consultant to provide services over an eight month period in exchange for 1,000,000 shares of common stock. The Company valued the 1,000,000 shares based on the market price on the agreement date of $0.0445 and will recognize $44,500 of consulting expense through the term of the agreement. On June 3, 2015 the Company issued the 1,000,000 shares of this agreement. The Company has recorded $7,265 of consulting expense as of June 30, 2015 related to this agreement.
On June 4, 2015, the Company entered into an agreement with a consultant to provide services over a six month period in exchange for 500,000 shares of common stock. The Company valued the 500,000 shares based on the market price on the agreement date of $0.0706 and will recognize $35,300 of consulting expense through the term of the agreement. On July 2, 2015 the Company issued the 500,000 shares of this agreement. The Company has recorded $5,015 of consulting expense as of June 30, 2015 related to this agreement.
Warrants:
In September, 2013, pursuant to convertible debenture, the Company issued 3,000,000 warrants to purchase common stock. These warrants have an initial exercise price of $0.0698 per share which is subject to adjustment and expire 5 years from the date of issuance (See Note 6).
In connection with above agreement dated May 7, 2015, the Company issued to the consultant, warrants for 3,379,158 common shares of the Company. The fair value of the warrants was determined using a Black-Scholes option pricing model with a stock price of $0.043, exercise price of $0.03, volatility of 397% based on the Company’s stock price, an expected term of 60 months based on the warrant and a risk free rate of 1.54%. The value of the warrants of $145,303 was recorded as additional paid in capital in the accompanying consolidated balance sheet, along with a prepaid expense of approximately $101,080 and stock based expense of approximately $44,223 for the year ended June 30, 2015.
In connection with above agreement dated May 21, 2015, the Company issued to the consultant warrants for 1,000,000 common shares of the Company. The fair value of the warrants was determined using a Black-Scholes option pricing model with a stock price of $0.0445, exercise price of $0.07, volatility of 397% based on the Company’s stock price, an expected term of 60 months based on the warrant and a risk free rate of 1.54%. The value of the warrants of $44,500 was recorded as additional paid in capital in the accompanying consolidated balance sheet, along with a prepaid expense of approximately $37,235 and stock based expense of approximately $7,265 for the year ended June 30, 2015.
As of June 30, 2015, there were 7,379,158 warrants outstanding and exercisable with expiration dates commencing September 2018 – May 2020. (See Note 6 and above).
The following table summarizes warrant activity for the years ended June 30, 2015 and 2014:
| Weighted | ||||||||
| Number of | Average | |||||||
| Shares | Price Per Share | |||||||
| Outstanding at June 30, 2013 | - | $ | - | |||||
| Issued | 3,000,000 | 0.07 | ||||||
| Exercised | - | - | ||||||
| Expired | - | - | ||||||
| Outstanding at June 30, 2014 | 3,000,000 | 0.07 | ||||||
| Issued | 4,379,158 | 0.04 | ||||||
| Exercised | - | - | ||||||
| Expired | - | - | ||||||
| Outstanding at June 30, 2015 | 7,379,158 | $ | 0.05 | |||||
| Exercisable at June 30, 2015 | 7,379,158 | $ | 0.05 | |||||
| Outstanding and Exercisable: | ||||||||
| Weighted average remaining contractual term | 4.21 | |||||||
| Aggregate intrinsic value | $ | 319,774 | ||||||
| F-23 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
NOTE 9 – COMMITMENTS AND CONTINGIENCIES
Legal Matters
From time to time, the Company may be involved in litigation relating to claims arising out of the Company’s operations in the normal course of business. The Company is presently in litigation with JMJ Financial Inc., a Florida corporation (“JMJ”), in the Circuit Court of Dade County, Florida. JMJ is claiming funds due under a convertible promissory note of Twenty Five thousand Dollars ($25,000.00). The Company is actively defending all allegations made by JMJ, and has lodged a counter claim against the plaintiff. The parties are currently negotiating for a possible settlement, and a mediation is expected to be scheduled in September or October 201 to determine a settlement. The Company does not believe the result of this litigation matter will have a material adverse effect on our financial conditions or results of operations.
Operating Agreements
In November 2009, the Company entered into a commercialization agreement whereby the Company agreed to pay royalties of 2% of net revenues. Additionally, the Company agreed to pay 5% of each and every license agreement subscribed for. The contract is cancellable at any time by either party. To date, no amounts are owed under the agreement.
Operating Leases
From July 2013 through April 30, 2015, the Company utilized office space at a certain location. There was no formal lease agreement and no amounts were paid, but the Company had accrued a liability as of April 30, 2015 of approximately $21,000 in anticipation of a month to month agreement retroactive to July 1, 2013 at approximately $1,000 per month. On May 1, 2015, the Company moved to new premises. The prior landlord verbally agreed that he would not be pursuing payment of any outstanding rent due, therefore the Company recorded a gain on settlement related to the accrued rent liability. On May 1, 2015, the Company entered into a month to month lease agreement with new landlord with a monthly rental fee of approximately $2,200 AUD and requiring a three month notice, by either party, to terminate agreement.
Rent expense for the years ended June 30, 2015 and 2014 were $3,719 and $11,016 respectively.
Settlement and Stipulation Agreement
In July 2014, the Company signed a term sheet and a Settlement and Stipulation Agreement (the "Settlement Agreement") with a third party purchaser (the "purchaser") to have that purchaser acquire certain portions of the Company’s liabilities to creditors (“Creditors”) in exchange for an obligation of the Company to issue shares of common stock to the purchaser, which shares of common stock would then be sold by the purchaser and 65% of the net proceeds, as defined in the agreement, distributed to the Creditors. The shares are to be freely traded shares issued pursuant to section 3(a)(10) of the Securities Act of 1933.
Under the terms of the Settlement Agreement, the variable quantity of common stock will be issued in tranches such that the purchaser would not own more than 9.99% of the outstanding shares of common stock at any time.
Under the above agreements, in May 2014 the Company also paid an expense fee of $25,000 in the form of a convertible promissory note. (See Note 6).
The purchaser entered into agreements through July 2014 with the Creditors to acquire $627,998 in liabilities of the Company and filed a complaint with the Second Judicial Circuit Court in Leon County, Florida seeking a judgment against the Company for such amount. A court order based on this complaint was issued on September 9, 2014, (the "court order date") resulting in the transfer of $627,998 in liabilities of the Company to the purchaser. In addition, upon entry of the order, the Company became obligated to issue the purchaser a fee of $50,000 worth of common stock priced at 75% of the average closing bid prices for the 10 days immediately preceding the date of the order. As a result of the purchased liabilities and purchaser fee, the Company became obligated to issue to the purchaser approximately $1,033,000 worth of common stock. These liabilities meet the criteria of stock settled debt under ASC 480 resulting in the recording of a liability premium of approximately $405,000 with a charge to interest expense on the court order date.
During the year ended June 30, 2015, the Company issued a total of 15,587,000 shares of common stock to the purchaser. As of June 30, 2015, the purchaser has sold all 15,587,000 shares of common stock which after fees, reduced the liability owed to the purchaser by $52,907. On January 30, 2015, as part of the Settlement and Lock-up agreement with the purchaser, this agreement was terminated and the Company reclassified the remaining principal outstanding debts and other liabilities of approximately $575,000. In addition, since this agreement was terminated, the Company wrote off the remaining premium of $310,000 and the fee of approximately $67,000 as a gain on debt settlement.
| F-24 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
Equity Purchase Agreement
On July 18, 2014 the Company executed an Equity Purchase Agreement (the "agreement") with an investor (the "investor") affiliated with the above purchaser. The Company may sell (put shares) from time to time, during the commitment period discussed below, up to $5,000,000 of the Company's common stock at a sale price equal to 90% of the market price. The market price is determined during a valuation period which is the 10 trading days immediately following the clearing date (the date when the put shares are deposited into the investor's brokerage account) associated with the applicable put notice. The valuation period may change based on any valuation events occurring, as defined in the agreement. The Company's right to sell to the investor and the investor's obligation to purchase shares is subject to certain restrictions, including a floor price, as defined in the agreement. Furthermore, on each closing date the number of shares then to be purchased shall not exceed that amount that when aggregated with all other shares beneficially owned by the investor would result in the investor owning more than 9.99% of the outstanding shares of common stock.
The commitment period is the earlier of the sale of $5,000,000 worth of shares or 24 months.
On July 18, 2014, Company entered into a Registration Rights Agreement with the investor. Pursuant to the terms of the Registration Rights Agreement, the Company is obligated to file a registration statement (the “Registration Statement”) with the SEC to cover the Registrable Securities within one hundred twenty (120) days of closing of an equity purchase. The Company must use its commercially reasonable efforts to cause the Registration Statement relating to the Registered Securities to become effective within five (5) business days after notice from the SEC that such Registration Statement may be declared effective, and keep the Registration Statement effective at all time prior to the termination of the Equity Purchase Agreement until the earliest of (i) date that is three months after the completion of the last Closing date (as defined in the Equity Purchase Agreement), (ii) the date when the investor may sell all Registered Securities under Rule 144 without volume limitations, or (iii) the date the investor no longer owns any of the Registered Securities (collectively, the “Registration Period”).
On July 18, 2014 the Company paid a $50,000 fee to the investor in the form of a $50,000 promissory note, non-interest bearing and due January 31, 2015. On January 30, 2015, the Company entered into a Settlement and Lock-up Agreement with the investor whereby the Company issued 10,000,000 shares of common stock as settlement of the $50,000 promissory note and a $25,000 convertible promissory note issued in connection with a Settlement and Stipulation Agreement dated May 2014 and accrued interest of $1,466. The Company valued the common stock at a price of $0.0025 per share based on the last private placement purchase price per share for a total value of $25,000 which resulted in the Company recording a gain of $51,466 as a result of this settlement. (See Notes 6 and 8).
NOTE 10 – RELATED PARTY TRANSACTIONS
Since inception, Propanc Health Group Corporation has conducted transactions with directors and director related entities. These transactions included the following:
As of June 30, 2015 and June 30, 2014, the Company owed certain directors a total of $79,416 and $161,975 respectively, for money loaned to the Company throughout the years. The loan balance owed at June 30, 2015 was not interest bearing (See Note 5).
As of June 30, 2015 and June 30 2014, the Company owed two directors a total of $35,108 and $60,350, respectively, related to expenses paid on behalf of the Company related to corporate startup costs and intellectual property (See Note 5).
On February 4, 2015, the Company entered into a Debt Settlement Agreement with some of these directors whereby the Company issued 33,259,350 and 17,654,470 shares of common stock as settlement of approximately $41,000 of the balance due to these directors. The Company valued the common stock at a price of $0.0025 per share based on the last private placement purchase price per share for a total value of $127,284 which resulted in the Company recording a loss of $86,455 as a result of these settlements.
NOTE 11 – CONCENTRATIONS AND RISKS
Concentration of Credit Risk
The Company maintains its cash in banks and financial institutions in Australia. Bank deposits in Australian banks are uninsured. The Company has not experienced any losses in such accounts through June 30, 2015.
Receivable Concentration
As of June 30, 2015 and 2014, the Company’s receivables were 100% related to reimbursements on GST taxes paid.
Product and Patent Concentration
As of June 30, 2015 the Company was undertaking preclinical activities for their lead product. The Company was also undertaking research to uncover the mechanism of action of their lead product in order to screen new compounds for development.
| F-25 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
The Company previously expanded by the filing of an international PCT patent application (No. PCT/AU2010/001403) directed to enhanced proenzyme formulations and combination therapies. The international PCT application has been based on previous provisional patent applications capturing the Company’s ongoing research and development in this area.
The Company received grant status in South Africa and more recently in Australia and New Zealand. In addition, the United States Patent and Trademark Office or USPTO and European Patent Office or EPO have made preliminary indications that key features of our technology are patentable. The Company is presently working towards securing a patent in each region, covering as many aspects of its technology as possible, while also actively seeking protection throughout Eastern Europe, Asia and South America. Individual countries and regions, include United States, Canada, Japan, Brazil, China, Mexico, Hong Kong, Singapore, Israel, Chile, Peru, Malaysia, Vietnam, Indonesia, Europe, Russia, India, and South Korea. The patent is granted in South Africa, Australia, and New Zealand.
Further provisional patent filings are also expected to be filed to capture and protect additional patentable subject matter that is identified, namely further enhanced formulations, combination treatments, use of recombinant products, modes of action and molecular targets.
Foreign Operations
As of June 30, 2015 and 2014, the Company's operations are based in Australia.
NOTE 12 - DERIVATIVE FINANCIAL INSTRUMENTS and FAIR VALUE MEASUREMENTS
Derivative Financial Instruments:
The Company applies the provisions of ASC Topic 815-40, Contracts in Entity’s Own Equity (“ASC Topic 815-40”), under which convertible instruments and warrants, which contain terms that protect holders from declines in the stock price (reset provisions), may not be exempt from derivative accounting treatment. As a result, warrants and embedded conversion options in convertible debt are recorded as a liability and are revalued at fair value at each reporting date. If the fair value of the warrants exceeds the face value of the related debt, the excess is recorded as change in fair value in operations on the issuance date. The Company has 3,000,000 warrants and $335,000 of convertible debt with repricing options and $87,500 of convertible debt with variable conversion pricing outstanding at June 30, 2015.
The Company calculates the estimated fair values of the liabilities for derivative instruments using the Black Scholes (“BSM”) option pricing model. The closing price of the Company’s common stock at June 30, 2015 was $0.0899. Volatility, expected remaining term and risk free interest rates used to estimate the fair value of derivative liabilities at June 30, 2015, are indicated in the table that follows. The volatility for initial valuation was based on comparative company’s methods since the Company’s stock is very thinly traded and historical volatility at June 30, 2015, the expected term is equal to the remaining term of the warrants and the risk free rate is based upon rates for treasury securities with the same term.
Warrants
| Initial Valuation | ||||||||||||
| September
30, 2013 | June 30, 2014 | June 30, 2015 | ||||||||||
| Volatility | 53 | % | 134 | % | 408 | % | ||||||
| Expected remaining term | 5 | 4.25 | 3.25 | |||||||||
| Risk-free interest rate | 0.4 | % | 0.47 | % | 1.63 | % | ||||||
| Expected dividend yield | None | None | None | |||||||||
Convertible Debt
| Initial Valuations | June 30, 2015 | |||||||
| Volatility | 216 - 377 | % | 408 | % | ||||
| Expected remaining term | 0.83 – 2.00 | 0.82 – 1.70 | ||||||
| Risk-free interest rate | 0.5 – 0.7 | % | 0.64 | % | ||||
| Expected dividend yield | None | None | ||||||
| F-26 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
Fair Value Measurements:
The Company measures and reports at fair value the liability for derivative instruments. The fair value liabilities for price adjustable warrants and embedded conversion options have been recorded as determined utilizing the BSM option pricing model. The following tables summarize the Company’s financial assets and liabilities measured at fair value on a recurring basis as of June 30, 2015 and 2014:
| Balance at | Quoted Prices | Significant | ||||||||||||||
| June | in Active | Other | Significant | |||||||||||||
| 30, | Markets for | Observable | Unobservable | |||||||||||||
| 2015 | Identical Assets | Inputs | Inputs | |||||||||||||
| (Level 1) | (Level 2) | (Level 3) | ||||||||||||||
| Embedded conversion option liabilities | $ | 780,281 | $ | — | $ | — | $ | 780,281 | ||||||||
| Fair value of liability for warrant derivative instruments | $ | 269,648 | $ | — | $ | — | $ | 269,648 | ||||||||
| Total | $ | 1,049,929 | $ | — | $ | — | $ | 1,049,929 | ||||||||
The following tables summarize the Company’s financial assets and liabilities measured at fair value on a recurring basis as of June 30, 2014:
| Balance at | Quoted Prices | Significant | ||||||||||||||
| June | in Active | Other | Significant | |||||||||||||
| 30, | Markets for | Observable | Unobservable | |||||||||||||
| 2014 | Identical Assets | Inputs | Inputs | |||||||||||||
| (Level 1) | (Level 2) | (Level 3) | ||||||||||||||
| Fair value of liability for warrant derivative instruments | $ | 158,244 | $ | — | $ | — | $ | 158,244 | ||||||||
The following is a roll forward for the years ended June 30, 2015 and 2014 of the fair value liability of price adjustable derivative instruments:
| Fair Value of | ||||
| Liability for | ||||
| Derivative | ||||
| Instruments | ||||
| Balance at June 30, 2013 | $ | - | ||
| Effects of foreign currency exchange rate changes | (2,519 | ) | ||
| Initial fair value of embedded conversion option derivative liability recorded as debt discount | 144,241 | |||
| Change in fair value included in statements of operations | 16,522 | |||
| Balance at June 30, 2014 | 158,244 | |||
| Effects of foreign currency exchange rate changes | (42,796 | ) | ||
| Initial fair value of embedded conversion option derivative liability recorded as debt discount | 392,500 | |||
| Initial fair value of embedded conversion option derivative liability recorded as change in fair value of ECO | 1,082,567 | |||
| Change in fair value included in statements of operations | (540,586 | ) | ||
| Balance at June 30, 2015 | $ | 1,049,929 | ||
NOTE 13 – SUBSEQUENT EVENTS
Subsequent to June 30, 2015, the Company received payment of the Secured Investor Note of $220,000 less OID of $20,000, that was issued on June 4, 2015. The Company received interest proceeds of $1,997 from the Secured Investor Note resulting in net cash proceeds of $201,997 received by the Company. The Secured Investor Note is convertible, at the option of the lender, to common stock of the Company at any time after the effective date at a price of $0.07 per share (See Note 6).
On July 14, 2015, the Company received payment of three Note Receivables of $352,500, that offset three of the Back-End Notes that were issued on May 19, 2015. Proceeds from the Note Receivables of $17,690 were paid directly to legal fees resulting in net cash proceeds of $334,810 received by the Company. As a result, these Back-End Notes are now eligible for conversion at a rate of 55% of the lowest trading bid price of the Company’s common stock for the ten prior trading days including the date upon which the conversion notice was received (See Note 6).
On July 15, 2015, the Company paid cash of $137,915 as payment in full of a convertible promissory note dated March 12, 2015. The repayment amount included principal of $104,000, accrued interest of $2,872 and a prepayment penalty of $31,043 (See Note 6).
On August 14, 2015, pursuant to a conversion notice, $20,500 of principal and interest was converted at $0.02365 into 866,796 shares of common stock (See Note 6).
| F-27 |
PROPANC HEALTH GROUP CORPORATION AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2015 and JUNE 30, 2014
On August 14, 2015, pursuant to a conversion notice, $20,802 of principal and interest was converted at $0.02365 into 879,585 shares of common stock (See Note 6).
On August 26, 2015, pursuant to a conversion notice, $26,068 of principal and interest was converted at $0.018425 into 1,414,843 shares of common stock (See Note 6).
On August 26, 2015, the Company issued 560,000 shares of common stock to a consultant. The Company valued the 560,000 shares based on the market price on the issuance date of $0.04.
On September 1, 2015, pursuant to a conversion notice, $25,723 of principal and interest was converted at $0.018425 into 1,396,108 shares of common stock (See Note 6).
On September 4, 2015, pursuant to a conversion notice, $15,648 of principal and interest was converted at $0.018425 into 849,263 shares of common stock (See Note 6).
On September 8, 2015, the Company issued 600,000 shares of common stock to a member of the Company’s Scientific Advisory Board. The Company valued the 600,000 shares based on the market price on the issuance date of $0.0369.
On September 16, 2015, pursuant to a conversion notice, $15,687 of principal and interest was converted at $0.018975 into 826,726 shares of common stock (See Note 6).
On September 18, 2015, pursuant to a conversion notice, $15,694 of principal and interest was converted at $0.017875 into 877,969 shares of common stock (See Note 6).
On September 22, 2015, pursuant to a conversion notice, $15,638 of principal and interest was converted at $0.01716 into 911,294 shares of common stock (See Note 6).
On September 24, 2015, (the “Issuance Date”), the Company entered into a Promissory Note with a Lender whereby the Lender loaned the Company $1,200,000 in exchange for the issuance of a Promissory Note (the “Note”). The Company issued a note with a principal amount of $1,200,000 to the Lender. The debenture has a maturity date of the earlier of: (i) the date on which the Company closes a subsequent equity offering in an amount greater than the principal amount of the Note; or (ii) June 24, 2016. On its face, the Note does not accrue any interest. In the event that the Lender does not proceed with a subsequent financing, beginning on the 46th day following the Issuance Date, the Note will have a one-time interest adjustment of $180,000 on the outstanding principal of the Note. Additionally, if the Lender does not wish to proceed with a subsequent financing, the Note will also be convertible into common stock at the lower of (i) $0.0346; or (ii) a twenty percent (20%) discount to the average of the two lowest closing prices of the common stock in the five trading days prior to the date of conversion. In connection with the Note, the Company entered into a Security Agreement dated September 24, 2015 with the Lender whereby the Company agreed to grant to Lender an unconditional and continuing, first priority security interest in all of the assets and property of the Company to secure the prompt payment, performance and discharge in full of all of Company’s obligations under the Note, provided, however that in the event the Lender does not proceed with a subsequent financing, any and all security interests shall be removed.
| F-28 |
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized in the State of Delaware, on March 24, 2016.
| PROPANC HEALTH GROUP CORPORATION | ||
| By: | /s/ James Nathanielsz | |
| James Nathanielsz | ||
| Chief Executive Officer (Principal Executive Officer) | ||
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement on Form S-1 has been signed by the following persons in the capacities indicated on March 24, 2016.
| Signature | Title | |
| /s/ James Nathanielsz | President, Chief Executive Officer, Chief Financial | |
| James Nathanielsz | Officer, Chief Operating Officer, Director, and Chairman (Principal Executive Officer, Principal Financial Officer, and Principal Accounting Officer) | |
| /s/ Julian Kenyon | Director | |
| Julian Kenyon |
106